Priming for Saline-Alkaline Tolerance in Rice: Current Knowledge and Future Challenges
Jiang Changjie, Liang Zhengwei, Xie Xianzhi
Review
Priming for Saline-Alkaline Tolerance in Rice: Current Knowledge and Future Challenges
Jiang Changjie1, Liang Zhengwei2, Xie Xianzhi1
(Rice Research Institute, Shandong Academy of Agricultural Sciences, Jinan 250100, China; Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun 130102, China)
Soil salinization and/or alkalization is a major constraint to crop production worldwide. Approximately 60% of the cultivated land is affected by salt, over half of which is alkalized.Alkaline soils are characterized by high alkalinity and typically high salinity, which creates a complex saline-alkaline (SA) stress that affects plant growth. Rice cultivation has been accepted as an important strategy for effective utilization of SA land if water is available for irrigation. Nevertheless, as a salt-sensitive plant, rice plants suffer severe SA-induced damage, which results in poor plant growth and grain yield. Various approaches have been employed to improve rice productivity in SA land. Among them, the priming technique has emerged as a powerful method for enhancing SA tolerance in rice plants. In this review, we summarized how SA stress damages rice plants, and then presented how priming treatment can mitigate such damage.
saline-alkaline stress; plant hormone; abscisic acid; reactive oxygen species; antioxidant; stress tolerance
It is estimated that more than7.32% of the land area (1.12 × 109hm2) is salt-affected (Wicke et al, 2011; Shahid et al, 2018; Liu and Wang, 2021). Approximately 60% of the cultivated land (9.0× 108hm2) suffers from salinization (3.4 × 108hm2, 23%) and alkalization (5.6 × 108hm2, 37%) (Shahid et al, 2018). Even worse, over 1.0× 106hm2of arable land continues to degrade every year because of increasing soil salinity (Xia et al, 2019; Liu and Wang, 2021), which poses a serious threat to the world crop production. In China, 3.5%–10.3% of the country’s land (3.4 × 107–9.9 × 107hm2) is salt-affected, and it is widely distributed in 17 Northern provinces (Li et al, 2021; Liu and Wang, 2021).A typical alkaline soil, for example, can be seen in the Songnen Plain of Northeast China, where 6.2% of the land area (7.66 × 106hm2) is alkalized (Wang et al, 2004; Wang et al, 2009), and alarmingly it continues to expand by 2.0 × 104hm2per year (Xiu, 2000; Yin et al, 2003; Yang et al, 2008; Zhang et al, 2013). The soils in this area are characterized by high alkalinity with soil pH ranging from 8.5 to 11.0 (Ma and Liang, 2007; Hossner, 2008),allowing only a few species of alkali- resistant halophytes to survive (Wang et al, 2004).
Soil alkalization commonly co-occurswith salinization, creating a complex saline-alkaline (SA) stress for plants. The high pH of SA soils not only directly damages plants but also causes deficiencies in nutritional minerals such as iron and phosphorus (Qadir et al, 2001; Rehman et al, 2012; Nandal and Hooda, 2013). Thus, SA stress is highly more damaging to plants than any single neutral saline stress (Campbell and Nishio, 2000; Hartung et al, 2002; Islam et al, 2011; Chen et al, 2012; Paz et al, 2012; Radi et al, 2012).
TheUnited Nations projected that the world’s population would reach 9.9 billion by 2050 (PRB, 2020), and FAO (2022) projections suggested a 140% increase in cereal production and 70% increase in total global food demand by 2050. However, it was estimated that approximately 20% of future increases in crop production will still come from land extensification (Gregory et al, 2002). Therefore, the development and effective agricultural use of the marginal SA land resources are key importance to meet the increasing food and feed demands of the ever-expanding human population and livestock production. Rice cultivation has been empirically accepted as an effective strategy for food production in SA land if water is available for irrigation (Leng et al, 2020; Ganapati et al, 2022). Nevertheless, rice is a salt-sensitive crop (Grattan et al, 2002; Islam et al, 2019), and is highly stressed under SA conditions, which results in poor grain yield and quality. Therefore, it is important to develop effective approaches to enhance rice plant resistance to SA stress, and thereby improve plant growth and productivity in SA fields.
Plants can become more tolerant to future stress exposure after experiencing biotic or abiotic stress, which is known as priming, preconditioning or hardening (Mauch-Mani et al, 2017; Llorens et al, 2020; Sharma et al, 2022; Shasmita et al, 2022). The primed state between the two stresses can last from days to months, and even to the next generation. Well-known examples include systemic acquired resistance and seed preconditioning (seed priming) (Beckers and Conrath, 2007; Goellner and Conrath, 2008; Jisha et al, 2013). Priming can also be triggered by various chemical compounds (chemical priming), such as plant hormones and their functional analogs (Bektas and Eulgem, 2015; Rhaman et al, 2020), reactive chemicals (Savvides et al, 2016; Kerchev et al, 2020), plant metabolites (Sako et al, 2021), and some inorganic salts (Kerchev et al, 2020). The primed plants are in a sensitized or potentiated physiological state that enables them to initiate a more rapid and/or effective resistance response to stress exposure.
In this review, we summarized the damaging effects of SA stress on rice plants, and presented recent research progress on the effects of priming on underlying the mechanisms of SA tolerance in rice plants.The aspects of molecular response of plants to SA stress (Rao et al, 2023) as well as agricultural use of SA land (Cao et al, 2021; Minhas et al, 2021) have been comprehensively summarized before, and are therefore not discussed further here.
Rice cultivation in SA paddy fields
Rice cultivation in SA land has been practiced for several decades worldwide. In the 1950s, Jilin Province in Northeastern China launched a strategy named ‘Reclamation of salt-alkali lands with rice cultivation’. After half a century of tremendous effort, the rice cultivation paddies reached2.7×107hm2in JilinProvince, and the average grain yield increased from 5 to 10 t/hm2in the reclaimed paddies in 2012 (Zhao et al, 2012). These exceptional results were achieved through comprehensive measures, including infrastructure construction, soil reclamation, and breeding of SA-tolerant rice varieties.
Remarkably, rice cultivation accelerates the pace of reclamation of SA soils (Wang et al, 2003b; Liang et al, 2008; Luo et al, 2019). Soil pH is decreased by 0.53 in the 0–20-cm layer between 1–3 years of rice cultivation (Huang et al, 2015). Similarly, rice planting for 5–10 years decreases pH value and total salt by 0.22‒0.24 and 0.07%‒0.10%, respectively, and increases the organic matter content by 3.9‒5.0 g/kg. Therefore, rice cultivation has been recommended and adopted as an effective strategy for the gradual reclamation of SA soils.
However, rice yields are often still below 5 t/hm2in SA paddies around the world. Maas and Grattan (1999) reported that the rice grain yield decreases by 12% per unit increase (dS/m) in electrical conductivity (EC) values above 3.0 dS/m. In alkaline soils with pH 9.8, the grain yield reduces by 25.1%, 37.2% and 67.6% in tolerant, semi-tolerant and sensitive rice genotypes, respectively (Rao et al, 2008). Consistently, the grain yield of rice reduces by 2 610 kg/hm2under SA conditions compared with that from improved land (Wang et al, 2003a). Thus, constant effort is required to construct a comprehensive system for high and stable yield production of rice in SA paddy fields.
SA stress damage to rice plants
SA stress severely damages rice plants over the entire growth period, including reduced seed germination rate, retarded plant growth, seedling death, decreased primary root length, lateral root number, tillering number, spikelet number per panicle and panicle weight, delayed heading and greatly reduced grain yield (Khan et al, 1997; Khan and Abdullah, 2003; Sun et al, 2004).
Plants growing in SA soil suffer from high ion toxicity (high salinity), alkalinity (high pH) and osmotic stress (Munns and Tester, 2008; Lv et al, 2013). A comparative analysis of the above three SA stress factors on rice seedling growth revealed that alkaline stress is the most important factor that inhibits rice seedling growth (Lv et al, 2013). This finding indicates that improving the alkaline stress tolerance of rice plants is criticalfor improving plant growth and productivity in SA soils.
Alkaline stress causes severe cellular damage, especially to the root system, as evidenced by the marked increase in malondialdehyde (MDA, an indicator of lipid peroxidation) content, cell damage, and induction of cell death-related genes, which in turn results in root cell death and subsequent wilting of the whole plant (Wei et al, 2015; Zhang et al, 2017). Evaluation of 25cultivars for their relative levels of tolerance (membership degree) to alkaline stress revealed that several root growth indices of rice seedlings, including root number, total root length, total root volume, average root diameter and total root surface area, are highly correlated with the relative levels of alkaline tolerance (Lv et al, 2015). These findings indicate that root growth is highly sensitive to alkaline stress and is closely correlated with tolerance level, and these root growth indices represent a set of simple and operational parameters for assessing alkaline stress tolerance in rice.
Plants produce reactive oxygen species (ROS) such as superoxide anions (O2·̄) and hydrogen peroxide (H2O2) in response to various biotic and abiotic stresses (Choudhury et al, 2017). These ROS at low levels function as important cellular signaling molecules in stress toleranceregulation (Müller et al, 2009; Baxter et al, 2014; Dietz et al, 2016; Willems et al, 2016; Mittler, 2017; Yu et al, 2017); but at high levels, they induce severe oxidative damage, including damage to cellular membranes (lipid peroxidation), DNA, RNA and proteins, which is irreversible, and even causes cell death (Choudhury et al, 2017; Mittler, 2017). The oxidative stress generated by ROS in plants is considered a major limitation to crop productivity (Sharma et al, 2017).
The root damage of rice seedlings under alkaline stress is associated with excessive accumulation of O2·̄ and H2O2in the roots, and reducing ROS accumulation by applying procyanidins (a potent antioxidant) to rice seedlings for 24 h before alkaline treatment significantly alleviates alkali-induced root damage and seedling growth inhibition (Zhang et al, 2017). Consistently, Guo et al (2014) showed that the alkaline tolerance of a rice mutant,(), is associated with reduced ROS accumulation under alkaline conditions, and Guan et al (2017) reported that over-expression of a chloroplast superoxide dismutase (SOD) gene () in rice plants increases survival rate, plant growth, and grain yield under alkaline conditions. These results strongly indicate that ROS accumulation is the most important factor in root cell damage and plant growth inhibition in rice seedlings under alkaline stress, and that alkaline stress tolerance is correlated with ROS-scavenging capacity.
Priming for enhanced tolerance to SA stress in rice
In this review, priming is defined as a time-limited pre-treatment of plants or seeds with a chemical or mild stress that leads to enhanced tolerance to subsequent stress exposure. Studies in which exogenous chemicals were continuously applied during stress treatment are not included in this review.Known priming chemicals and their effects on SA stress tolerance in rice plants are summarized in Table 1.
Impact of priming with plant hormones
The plant hormone abscisic acid (ABA) plays a key role in plant adaptive response to various environmental stresses (Mittler and Blumwald, 2015; Jones, 2016; Sah et al, 2016). Wei et al (2015) transiently treated rice seedlings with ABA (0, 10 or 50mmol/L) by root-drenching for 24 h and then, the seedlings were exposed to alkaline stress (15 mmol/L Na2CO3, pH 10.87). Measurement of seedling growth revealed that ABA pretreatment significantly improves seedling survival rate, root growth and biomass compared with the control treatment (0mmol/L ABA) (Wei et al, 2015). These results demonstrated that ABA has a potent priming effect on alkaline stress tolerance in rice. Physiologically, ABA pretreatment increases SOD, catalase (CAT), ascorbate peroxidase (APX) and peroxidase (POD) activities, but reduces alkali- and paraquat-induced accumulation of O2·̄and H2O2, electrolyte leakage (an indicator of membrane and cell injury) and Na+/K+ratio (Liu et al, 2019). In addition, expression levels of the stress tolerance- related genes,,,andare superinduced by ABA pretreatment under alkaline conditions (Liu et al, 2019). These results indicate that ABA priming increases alkaline tolerance by upregulating the antioxidant defense system and stress tolerance-related genes, and maintaining an appropriate Na+/K+ratio and cellular membrane integrity.
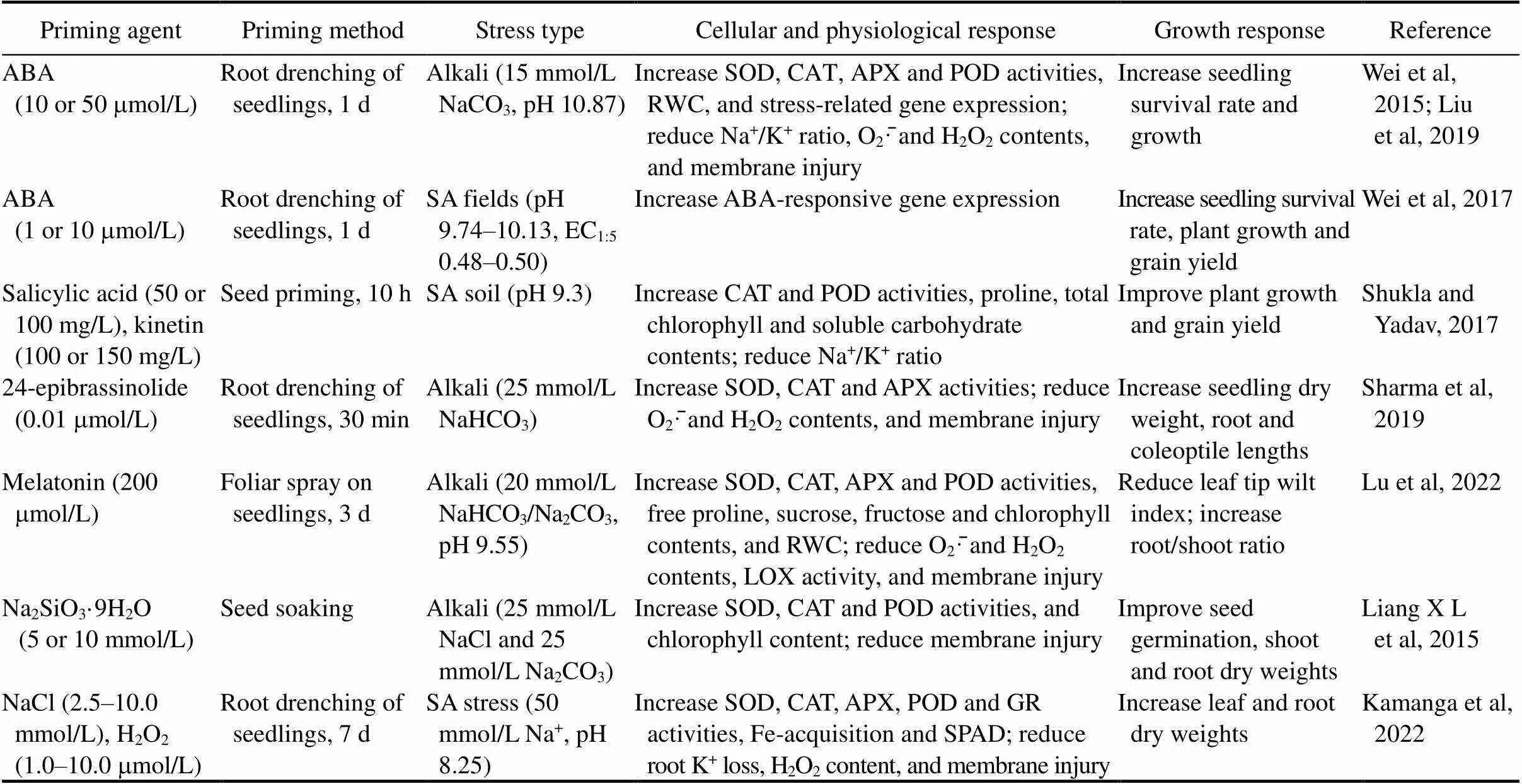
Table 1. Known priming chemicals and their effects on saline-alkaline (SA) stress tolerance in rice plants.
ABA, Abscisic acid; SOD, Superoxide dismutase; CAT, Catalase; APX, Ascorbate peroxidase; EC, Electrical conductivity; LOX, Lysyl oxidase; POD, Peroxidase; RWC, Relative water content; GR, Glutathion reductase; SPAD, Soil and plant analyzer development.
The priming effect of ABA was further corroborated in a 3-year field trial (Wei et al, 2017). Rice seedlings were treated with ABA (0, 1 and 10mmol/L) by root-drenching for 24 h before transplanting to paddy fields with two different SA levels, pH 10.1/EC1:50.50 and pH 9.7/EC1:50.48. The results showed that ABA-priming significantly reduces the seedling death by 11%–17% and leaf withering by 22%–50%, and significantly improves subsequent plant growth and final grain yield by 8%–55% compared with the control treatment (0mmol/L ABA). A more profound effect was observed in the heavier SA field. The magnitude of the ABA effect on grain yield varies by experimental years, but is consistently significant across all the three years in treated and control SA fields. Notably, these beneficial effects of ABA were obtained with only a single prior treatment (priming). These results confirm that there is a strong priming effect of ABA on SA stress tolerance of rice plants in SA fields, which may provide an effective and practical approach to improve the rice production in SA fields.
Furthermore, the expression levels of the ABA- responsive genesandare highly induced by ABA treatment, and the upregulated levels are persisted until at least 8 d after transplanting, which indicates a long-lasting effect of ABA priming in the SA fields (Liu X L et al, 2022). The alleviating effects of ABA on alkaline and SA stress were also confirmed by RNAi-mediated knockdown of(-kd), a key ABA catabolic gene, which results in higher endogenous ABA levels and survival rates under hydroponic alkaline conditions and in pot trials using soil from SA fields (Liu X L et al, 2022).
The priming impacts of plant hormones are also shown by salicylic acid, kinetin (Shukla and Yadav, 2017), brassinosteroid (Sharma et al, 2019), and melatonin (Lu et al, 2022). Seed priming with salicylic acid (50 or 100 mg/L) and kinetin (100 or 150 mg/L) upregulates CAT and POD activities, chlorophyll and soluble carbohydrate contents and K+/Na+ratio, which leads to improved plant growth and grain yield under SA soil conditions (pH 9.3) (Shukla and Yadav, 2017). Sharma et al (2019) found that short-term root-drenching of rice seedlings for 10 h with 0.01mmol/L of 24-epibrassinolide (a brassinosteroid) significantly improves seedling survival and growth, and grain yield under alkaline stress (25 mmol/L NaHCO3) by upregulating SOD, CAT and APX activities, and reducing O2·̄ and H2O2contents and membrane injury. Furthermore, it was recently reported that foliar spray of melatonin (200mmol/L) on rice seedlings for 3 d reduces leaf wilt index and increases root/shoot ratio under alkaline stress conditions (20 mmol/L NaHCO3/Na2CO3, pH 9.55) (Lu et al, 2022). Physiologically, melatonin priming upregulates SOD, CAT, APX and POD activities, and compatible osmolytes including proline, sucrose and fructose, chlorophyll content, and leaf relative water content (RWC), but reducesO2·̄ and H2O2contents, lysyl oxidase activity, and membrane injury.
Impact of priming with inorganic salts
Silicon (Si), though not an essential nutrient, has many beneficial effects on plants, especially under various biotic and abiotic stresses (Liang Y C et al, 2015; Haynes, 2017). Rice accumulates a large amount of Si, reaching as much as 4.2% of the shoot dry weight (Guntzer et al, 2012). Liang X L et al (2015) reported that seed priming with Na2SiO3·9H2O significantly improves rice seed germination and seedling growth under SA stress. The improvement effects of Si are accompanied by activation of the antioxidant enzymes SOD, POD and CAT, and reduction of MDA content in rice seedlings. These results indicated that Si plays a pivotal role in SA tolerance of rice seedlings by activating antioxidant mechanisms. A similar effect of Si was also observed in maize, in which seed priming with Si for 12 h significantly improves maize plant growth under alkaline stress with Na2CO3concentration less than 75 mmol/L (Abdel Latef and Tran, 2016). It was further shown that the effect of Si priming is correlated with increased antioxidant enzyme activities (SOD, CAT and POD), leaf RWC, and levels of photosynthetic pigments, osmolytes and K+/Na+(Abdel Latef and Tran, 2016).
Recently, Kamanga et al (2022) reported that pre-exposure of rice seedlings to low concentrations of NaCl (2.5–10.0 mmol/L) and H2O2(1.0–10.0mmol/L) for 7 d results in cross-tolerance to SA stress (50 mmol/L Na+, pH 8.25), as evidenced by improved seedling growth, and maintained chlorophyll content and pH of the growth medium. It was revealed that the enhanced SA tolerance is associated with enhanced Fe acquisition, and improved Na+and ROS homeostasis.
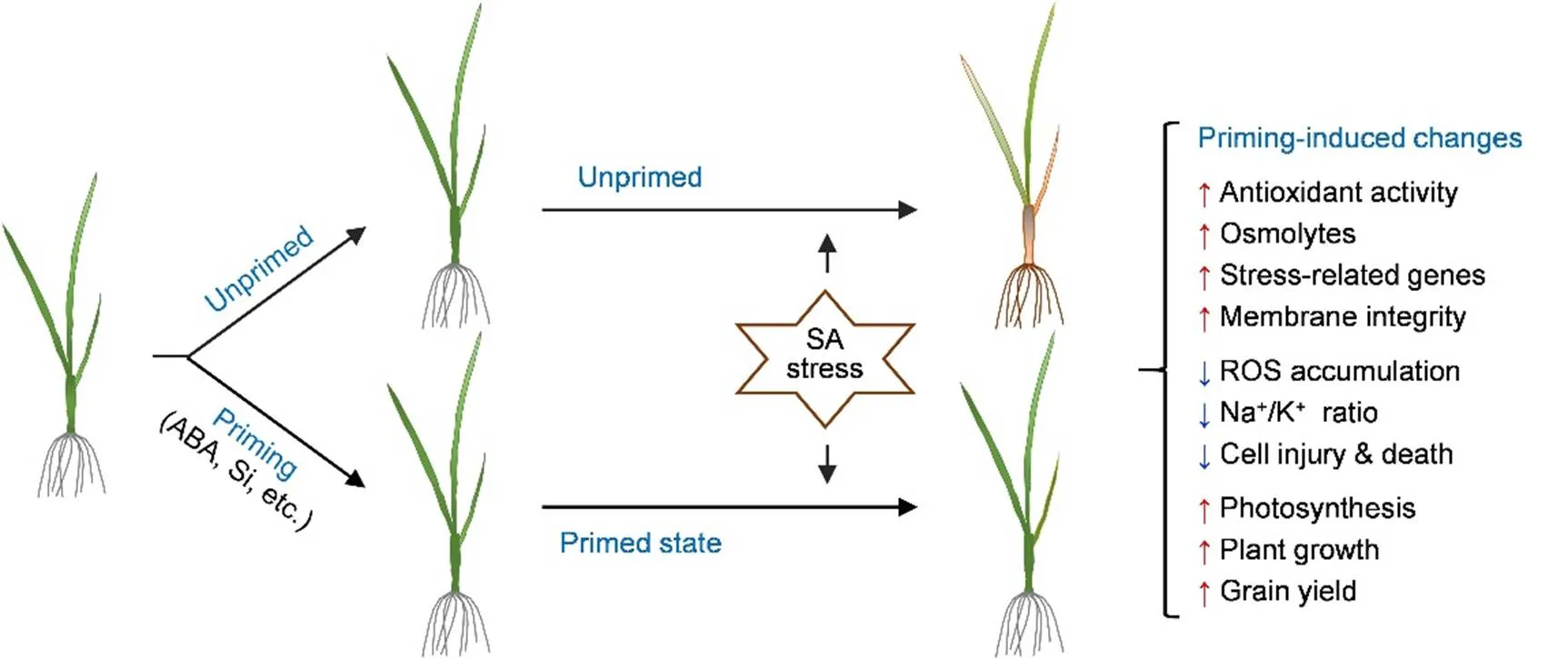
Fig. 1. Schematic illustration of priming-induced relative changes in physiological and cellular characteristics and growth traits in rice plants under saline-alkaline (SA) stress.
ABA, Abscisic acid; Si, Silicon; SA, Saline-alkaline; ROS, Reactive oxygen species.
Up (red)- and down (blue)-pointing arrows indicate up- and down-changes relative to the unprimed control, respectively.
Perspectives
Priming is a cost-effective and environmentally friendly agro-biological technique for improving stress tolerance and plant growth in various crop plants (Aranega-Bou et al, 2014; Sako et al, 2021). Each priming agent has its own appropriate use and effect. For example, ABA may be unsuitable for seed priming treatment as it generally inhibits seed germination, whereas seed priming with Si improves seed germination. Therefore, it is important and interesting to assess the effect of combining different priming agents for greater efficacy in future studies.
As depicted in Fig. 1 and summarized in Table 1, priming can potentiate rice plants to increase antioxidant activity, osmolyte content, stress-related gene expression, and membrane integrity upon exposure to SA stress, thereby maintaining redox and ionic homeostasis, protecting cells from injury and death, and eventually leading to better plant growth and grain yield under SA stress conditions. The most important and attractive advantage of priming may be its long-lasting effect on multiple types of abiotic stress. For example, pretreatment with exogenous ABA can enhance tolerance to various stresses, such as drought, low or high temperature, and high salinity and alkalinity, in a wide range of plant species (Jones, 2016; Sah et al, 2016). Additionally, a single short- term treatment with ABA results in marked improvement in plant growth and grain yield under various stress conditions (Gurmani et al, 2006, 2011, 2013; Wei et al, 2015, 2017). These findings strongly indicate that ABA serves as a potent priming agent, and the recent discoveries of various functional analogs of ABA and inhibitors of ABA metabolism (Dejonghe et al, 2018; Hewage et al, 2020) will undoubtedly facilitate the practical application of ABA pathway-targeted priming to improve crop productivities in SA fields.
Alkalinity causes much more severe damage to rice seedlings, particularly to the root system, compared with osmotic stress and high salinity (Lv et al, 2013). Therefore, improving the alkaline stress tolerance is a top priority for improving rice plant growth and productivity in SA soils. Alkaline stress, like many other stresses, causes hyper-accumulation of ROS and cellular membrane damage, which in turn results in cell injury and cell death (Zhang et al, 2017). As shown in Table 1, the priming effects of different chemicals are all associated with increased antioxidant enzyme activity and reduced ROS accumulation. In addition, maintaining ROS homeostasis is a key priming effect in various plant species under various stresses (Choudhury et al, 2017). Thus, suppressing excess ROS accumulation will be the key for successful priming treatments.
ACKNOWLEDGEMENTS
This study was supported by the Science and Technology Innovation Project of Shandong Academy of Agricultural Sciences, China (Grant No. CXGC2022F02), and the Agricultural Variety Improvement Project of Shandong Province, China (Grant No. 2019LZGC003). We thank Mallory Eckstut from Liwen Bianji (Edanz) (www.liwenbianji.cn) for English improvement of this manuscript.
Abdel Latef A A, Tran L S P. 2016. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress., 7: 243.
Aranega-Bou P, de la O Leyva M, Finiti I, García-Agustín P, González-Bosch C. 2014. Priming of plant resistance by natural compounds: Hexanoic acid as a model., 5: 488.
Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signalling., 65(5): 1229–1240.
Beckers G J M, Conrath U. 2007. Priming for stress resistance: From the lab to the field., 10(4): 425–431.
Bektas Y, Eulgem T. 2015. Synthetic plant defense elicitors., 5: 804.
Campbell S A, Nishio J N. 2000. Iron deficiency studies of sugar beet using an improved sodium bicarbonate-buffered hydroponic growth system., 23(6): 741–757.
Cao X F, Sun B, Chen H B, Zhou J M, Song X W, Liu X J, Deng X D, Li X J, Zhao Y G, Zhang J B, Li J Y. 2021. Approaches and research progresses of marginal land productivity expansion and ecological benefit improvement in China., 36(3): 336–348. (in Chinese with English abstract)
Chen S S, Xing J J, Lan H Y. 2012. Comparative effects of neutral salt and alkaline salt stress on seed germination, early seedling growth and physiological response of a halophyte species., 11(40): 9572–9581.
Choudhury F K, Rivero R M, Blumwald E, Mittler R. 2017. Reactive oxygen species, abiotic stress and stress combination., 90(5): 856–867.
Dejonghe W, Okamoto M, Cutler S R. 2018. Small molecule probes of ABA biosynthesis and signaling., 59(8): 1490–1499.
Dietz K J, Turkan I, Krieger-Liszkay A. 2016. Redox- and reactive oxygen species-dependent signaling into and out of the photo- synthesizing chloroplast., 171(3): 1541–1550.
FAO (Food and Agriculture Organization). 2022. How to feed the World in 2050. [2023-01-01]. https://www.fao.org/fileadmin/ templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf.
Ganapati R K, Naveed S A, Zafar S, Wang W S, Xu J L. 2022. Saline-alkali tolerance in rice: Physiological response, molecular mechanism, and QTL identification and application to breeding., 29(5): 412–434.
Goellner K, Conrath U. 2008. Priming: It’s all the world to induced disease resistance., 121(3): 233–242.
Grattan S R, Zeng L H, Shannon M C, Roberts S R. 2002. Rice is more sensitive to salinity than previously thought., 56(6): 189–195.
Gregory P J, Ingram J S I, Andersson R, Betts R A, Brovkin V, Chase T N, Grace P R, Gray A J, Hamilton N, Hardy T B, Howden S M, Jenkins A, Meybeck M, Olsson M, Ortiz-Monasterio I, Palm C A, Payn T W, Rummukainen M, Schulze R E, Thiem M, Valentin C, Wilkinson M J. 2002. Environmental consequences of alternative practices for intensifying crop production., 88(3): 279–290.
Guan Q J, Liao X, He M L, Li X F, Wang Z Y, Ma H Y, Yu S, Liu S K. 2017. Tolerance analysis of chloroplast OsCu/Zn-SOD overexpressing rice under NaCl and NaHCO3stress., 12(10): e0186052.
Guntzer F, Keller C, Meunier J D. 2012. Benefits of plant silicon for crops: A review., 32(1): 201–213.
Guo M X, Wang R C, Wang J, Hua K, Wang Y M, Liu X Q, Yao S G. 2014. ALT1, a Snf2 family chromatin remodeling ATPase, negatively regulates alkaline tolerance through enhanced defense against oxidative stress in rice., 9(12): e112515.
Gurmani A R, Bano A, Salim M. 2006. Effect of growth regulators on growth, yield and ions accumulation of rice (L.) under salt stress., 38(5): 1415–1424.
Gurmani A R, Bano A, Khan S U, Din J, Zhang J L. 2011. Alleviation of salt stress by seed treatment with abscisic acid (ABA), 6-benzylaminopurine (BA) and chlormequat chloride (CCC) optimizes ion and organic matter accumulation and increases yield of rice (L.)., 5(10): 1278–1285.
Gurmani A R, Bano A, Ullah N, Khan H, Jahangir M, Flowers T J. 2013. Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice ()., 7: 1219–1226.
Hartung W, Leport L, Ratcliffe R G, Sauter A, Duda R, Turner N C. 2002. Abscisic acid concentration, root pH and anatomy do not explain growth differences of chickpea (L.) and lupin (L.) on acid and alkaline soils., 240(1): 191–199.
Haynes R J. 2017. Significance and role of Si in crop production., 146: 83–166.
Hewage K A H, Yang J F, Wang D, Hao G F, Yang G F, Zhu J K. 2020. Chemical manipulation of abscisic acid signaling: A new approach to abiotic and biotic stress management in agriculture., 7(18): 2001265.
Hossner L R. 2008. Field pH.: Chesworth W. Encyclopedia of Soil Science. Dordrecht, the Netherlands: Springer: 271–272.
Huang L H, Liang Z W, Suarez D L, Wang Z C, Wang M M, Yang H Y, Liu M. 2015. Impact of cultivation year, nitrogen fertilization rate and irrigation water quality on soil salinity and soil nitrogen in saline-sodic paddy fields in Northeast China., 154(4): 632–646.
Islam F, Wang J, Farooq M A, Yang C, Jan M, Mwamba T M, Hannan F, Xu L, Zhou W J. 2019. Rice responses and tolerance to salt stress: Deciphering the physiological and molecular mechanisms of salinity adaptation.: Hasanuzzaman M, Fujita M, Nahar K, Biswas J K. Advance in Rice Research for Abiotic Stress Tolerance. Cambridge, UK: Woodhead Publishing: 791–819.
Islam M S, Akhter M, El-Sabagh A, Liu L Y, Nguyen N T, Ueda A, Masaoka Y, Saneoka H. 2011. Comparative studies on growth and physiological responses to saline and alkaline stresses of Foxtail millet (L.) and Proso millet (L.)., 5(10): 1269–1277.
Jisha K C, Vijayakumari K, Puthur J T. 2013. Seed priming for abiotic stress tolerance: An overview., 35(5): 1381–1396.
Jones A M. 2016. A new look at stress: Abscisic acid patterns and dynamics at high-resolution., 210(1): 38–44.
Kamanga R M, Oguro S, Nampei M, Ueda A. 2022. Acclimation to NaCl and H2O2develops cross tolerance to saline-alkaline stress in Rice (L.) by enhancing fe acquisition and ROS homeostasis., 68(3): 342–352.
Kerchev P, van der Meer T, Sujeeth N, Verlee A, Stevens C V, Van Breusegem F, Gechev T. 2020. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants., 40: 107503.
Khan M S A, Hamid A, Karim M A. 1997. Effect of sodium chloride on germination and seedling characters of different types of rice (L.)., 179(3): 163–169.
Khan M A, Abdullah Z. 2003. Salinity-sodicity induced changes in reproductive physiology of rice () under dense soil conditions., 49(2): 145–157.
Leng C X, Zheng F Y, Zhao B P, Li H Y, Wang Y J. 2020. Advances on alkaline tolerance of rice., 36(11): 103–111. (in Chinese with English abstract)
Li F L, Luo C K, Lu X P, Tian L, Li P F. 2021. Current status and prospect of research on physiology and genetic mechanism of alkali tolerance in rice., 22(2): 283–292. (in Chinese with English abstract)
Liang X L, Fang S M, Ji W B, Zheng D F. 2015. The positive effects of silicon on rice seedlings under saline-alkali mixed stress., 46(17): 2127–2138.
Liang Y C, Nikolic M, Bélanger R, Gong H J, Song A L. 2015. History and introduction of silicon research.: Silicon in Agriculture. Dordrecht, the Netherlands: Springer: 1–18.
Liang Z W, Wang Z C, Ma H Y, Yang F, Huang L H, Kong X J, Yan C, Liu M, Wang M M, Qi C Y. 2008. The progress in improvement of high pH saline-alkali soil in the Songnen Plain by stress tolerant plants., 30: 517–528. (in Chinese with English abstract)
Liu H P, Able A J, Able J A. 2022. Priming crops for the future: Rewiring stress memory., 27(7): 699–716.
Liu L L, Wang B S. 2021. Protection of halophytes and their uses for cultivation of saline-alkali soil in China., 10(5): 353.
Liu X L, Zhang H, Jin Y Y, Wang M M, Yang H Y, Ma H Y, Jiang C J, Liang Z W. 2019. Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance-related genes., 438(1): 39–55.
Liu X L, Xie X Z, Zheng C K, Wei L X, Li X W Jin Y Y, Zhang G H, Jiang C J, Liang Z W. 2022. RNAi-mediated suppression of the abscisic acid catabolism geneincreases abscisic acid content and tolerance to saline-alkaline stress in rice (L.)., 10(2): 354–367.
Llorens E, González-Hernández A I, Scalschi L, Fernández-Crespo E, Camañes G, Vicedo B, García-Agustín P. 2020. Priming mediated stress and cross-stress tolerance in plants: Concepts and opportunities.: Hossain M A, Liu F L, Burritt D J, Fujita M, Huang B R. Priming-mediated Stress and Cross-stress Tolerance in Crop Plants. UT, USA: Academic Press: 1–20.
Lu X P, Min W F, Shi Y F, Tian L, Li P F, Ma T L, Zhang Y X, Luo C K. 2022. Exogenous melatonin alleviates alkaline stress by removing reactive oxygen species and promoting antioxidant defence in rice seedlings., 13: 849553.
Luo C K, Tian L, Bi J T, Xiao G J. 2019. Effects of rice planting years on saline-alkali soil trace elements, rice yield and quality., 28(8): 1577–1584.
Lv B S, Li X W, Ma H Y, Sun Y, Wei L X, Jiang C J, Liang Z W. 2013. Differences in growth and physiology of rice in response to different saline-alkaline stress factors., 105(4): 1119–1128.
Lv B S, Ma H Y, Li X W, Wei L X, Lv H Y, Yang H Y, Jiang C J, Liang Z W. 2015. Proline accumulation is not correlated with saline-alkaline stress tolerance in rice seedlings., 107(1): 51–60.
Ma H Y, Liang Z W. 2007. Effects of different soil pH and soil extracts on the germination and seedling growth of., 24(2): 181–188. (in Chinese with English abstract)
Maas E V, Grattan S R. 1999. Crop yields as affected by salinity.: Skaggs R W, van Schilfgaarde J. Agrcicultural Drainage. WI, USA: American Society of Agronomy: 38: 55–110.
Mauch-Mani B, Baccelli I, Luna E, Flors V. 2017. Defense priming: An adaptive part of induced resistance., 68: 485–512.
Minhas P S, Yadav R K, Sharma P C. 2021. Managing salt-affected soils for sustainable agriculture. New Delhi, India: ICAR.
Mittler R, Blumwald E. 2015. The roles of ROS and ABA in systemic acquired acclimation., 27(1): 64–70.
Mittler R. 2017. ROS are good., 22(1): 11–19.
Müller K, Linkies A, Vreeburg R A M, Fry S C, Krieger-Liszkay A, Leubner-Metzger G. 2009.cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth., 150(4): 1855–1865.
Munns R, Tester M. 2008. Mechanisms of salinity tolerance., 59: 651–681.
Nandal M, Hooda R. 2013. Salt tolerance and physiological response of plants to salinity: A Review., 4(10): 45–67.
Paz R C, Rocco R A, Reinoso H, Menéndez A B, Pieckenstain F L, Ruiz O A. 2012. Comparative study of alkaline, saline, and mixed saline-alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of., 31(3): 448–459.
PRB (Population Reference Bureau). 2020. The 2020 World population data sheet. [2023-01-01]. https://interactives.prb.org/ 2021-wpds/.
Qadir M, Schubert S, Ghafoor A, Murtaza G. 2001. Amelioration strategies for sodic soils: A review., 12(4): 357–386.
Radi A A, Abdel-Wahab D A, Hamada A M. 2012. Evaluation of some bean lines tolerance to alkaline soil., 2(1): B18–B27.
Rao P S, Mishra B, Gupta S R, Rathore A. 2008. Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes., 127(3): 256–261.
Rao Y, Peng T, Xue S W. 2023. Mechanisms of plant saline-alkaline tolerance., 281: 153916.
Rehman H U, Aziz T, Farooq M, Wakeel A, Rengel Z. 2012. Zinc nutrition in rice production systems: A review., 361(1): 203–226.
Rhaman M S, Imran S, Rauf F, Khatun M, Baskin C C, Murata Y, Hasanuzzaman M. 2020. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress., 10(1): 37.
Sah S K, Reddy K R, Li J X. 2016. Abscisic acid and abiotic stress tolerance in crop plants., 7: 571.
Sako K, Nguyen H M, Seki M. 2021. Advances in chemical priming to enhance abiotic stress tolerance in plants., 61(12): 1995–2003.
Savvides A, Ali S, Tester M, Fotopoulos V. 2016. Chemical priming of plants against multiple abiotic stresses: Mission possible?, 21(4): 329–340.
Shahid S A, Zaman M, Heng L E. 2018. Soil salinity: Historical perspectives and a world overview of the problem.: Zaman M, Shahid S A, Heng L E. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. Cham, Switzerland: Springer International Publishing: 43–53.
Sharma M, Gupta S K, Deeba F, Pandey V. 2017. Effects of reactive oxygen species on crop productivity: An overview.: Singh V P, Singh S, Tripathi D K, Prasad S M, Chauhan D K. Reactive Oxygen Species in Plants: Boon or Bane‐Revisiting the Role of ROS. Chichester, UK: John Wiley & Sons Ltd: 117–136.
Sharma M, Mahajan P, Singh H P, Batish D R, Kohli R K. 2019. 24-Epibrassinolide pre-treatment reduces alkaline-induced oxidative stress in red rice seedlings., 26(22): 23192–23197.
Sharma M, Kumar P, Verma V, Sharma R, Bhargava B, Irfan M. 2022. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects., 179: 10–24.
Shasmita B B S, Mohapatra P K, Naik S K, Mukherjee A K. 2022. Biopriming for induction of disease resistance against pathogens in rice., 255(6): 113.
Shukla A K, Yadav A K. 2017. Response of PGRS on growth, biochemical changes and yield of rice (L.) under sodic soil., 10(34): 7297–7300.
Sun T, Du Z Y, Zhang R Z, Meng F X, Yang J, Ma J Y. 2004. Effectof salinity-alkalinity stress on tillering and yield of rice., 28(6): 597–605. (in Chinese with English abstract)
Wang C Y, Wu Z J, Shi Y I, Wang R Y. 2004. The resource of saline soil in the Northeast China., 35: 643–647. (in Chinese with English abstract)
Wang L, Seki K, Miyazaki T, Ishihama Y. 2009. The causes of soil alkalinization in the Songnen Plain of Northeast China., 7(3): 259–270.
Wang Z C, Li Q S, Li X J, Song C C, Zhang G X. 2003a. Sustainable agriculture development in saline-alkali soil area of Songnen Plain, Northeast China., 13(2): 171–174.
Wang Z C, Sun C Z, Li X J, Shao X W. 2003b. Integrated technique model of rice production on saline-alkali land., 1: 56–59.
Wei L X, Lv B S, Wang M M, Ma H Y, Yang H Y, Liu X L, Jiang C J, Liang Z W. 2015. Priming effect of abscisic acid on alkaline stress tolerance in rice (L.) seedlings., 90: 50–57.
Wei L X, Lv B S, Li X W, Wang M M, Ma H Y, Yang H Y, Yang R F, Piao Z Z, Wang Z H, Lou J H, Jiang C J, Liang Z W. 2017. Priming of rice (L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields., 203: 86–93.
Wicke B, Smeets E, Dornburg V, Vashev B, Gaiser T, Turkenburg W, Faaij A. 2011. The global technical and economic potential of bioenergy from salt-affected soils., 4(8): 2669–2681.
Willems P, Mhamdi A, Stael S, Storme V, Kerchev P, Noctor G, Gevaert K, van Breusegem F. 2016. The ROS wheel: Refining ROS transcriptional footprints., 171(3): 1720–1733.
Xia J B, Ren J Y, Zhang S Y, Wang Y H, Fang Y. 2019. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China., 349: 25–35.
Xiu L N. 2000. The alkili-saline land and agricultural sustainable development of the western Songnen Plain in China., 1: 008.
Yang C W, Shi D C, Wang D L. 2008. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte(Bge.)., 56(2): 179–190.
Yin H N, Tang Z, Lu F. 2003. Analysis of eco-environment degradation mechanism in the west of Northeast Plain in China during the last 100 years., 10(4): 190–192.
Yu S X, Feng Q N, Xie H T, Li S, Zhang Y. 2017. Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato., 17(1): 76.
Zhang H, Liu X L, Zhang R X, Yuan H Y, Wang M M, Yang H Y, Ma H Y, Liu D, Jiang C J, Liang Z W. 2017. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice (L.)., 8: 1580.
Zhang X G, Huang B, Liang Z W, Zhao Y C, Sun W X, Hui W Y. 2013. Study on salinization characteristics of surface soil in western Songnen Plain., 45(2): 332–338. (in Chinese with English abstract)
Zhao G C, Qi C Y, Hou L G, Ma W, Sui P J, Liu L, Guo X M, Sun H J. 2012. Progress and prospect of rice production in Jilin Province at soda saline-alkaline land., 43(6): 673–680. (in Chinese with English abstract)
Copyright © 2023, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2023.05.003
19 March 2023;
5 May 2023
Jiang Changjie (cypa44@hotmail.com)
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury
- Effect of GW8 Gene Editing on Appearance Quality of Erect-Panicle Type (dep1) Japonica Rice
- Transcriptome Analysis of oserf922 Mutants Reveals New Insights into Rice Blast Resistance
- ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice
- LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice
- Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa

