CF-FM蝙蝠听中枢神经元处理行为相关声信号的神经机制*
马金哲 韩洋洋 姚一婷 付子英 陈其才 唐 佳
(华中师范大学生命科学学院,遗传调控与整合生物学湖北省重点实验室,武汉 430079)
Animal behaviors and their physiological status are always under the control of the nervous system.The nervous system can rapidly acquire, process and integrate sensory information, and adjust physiological status to an optimal point. All these adjustments, led by the nervous system, give animals the ability to adapt to their environment. Different types of neurons may play different roles in various behavioral tasks[1], that is, they appear to have a division of labor and cooperation. But no matter what type of neuron, the response characteristics of the nervous system will directly reflect the animal’s behavioral changes. In turn, studying the behavior of an animal is also important for understanding the coding strategies of the nervous system[2].
Bat echolocation is one of the most important discoveries of adaptive behavior in animals. With this highly specialized echolocation system, bats can occupy a unique ecological niche―the night sky, by which bats can avoid competing with most birds.During flight, echolocating bats can accurately analyze the differences between the emitted pulse and the returning echo to determine the physical properties of the surrounding external environment[3‑5]. To enable the returning echoes to convey maximum information about a target, bats are able to adjust the parameters of their emitted pulse at any time[6‑7]. Through precise processing of information in an echo by the auditory system, bats can form a distance map of their target in their auditory cortex (AC), thereby achieving the purpose of “seeing the world by hearing”. Inspired by bat’s echolocation capabilities, researchers have developed many artificial sonar devices, such as “human echolocation systems” and “electronic bat ears”, but the precision and sensitivity of these devices are far below that of echolocating bats. In recent years, the highly specialized echolocation system of bats and its regulatory mechanisms have been extensively studied.The goal of this article is to integrate information gained from this research to better understand the neural mechanisms in the central auditory system for processing behavior‑related acoustic signals of echolocating bats. We hope it can offer some new points of reference for related research in the future.
1 The function of the central auditory system in the echolocation task
To complete the echolocation and preying task successfully, the central auditory system of echolocating bats must satisfy the following requirements to adapt the bats’ behavior. Firstly, the central auditory system must quickly identify and process species‑specific sound signal, which allows a bat to extract meaningful biological sound information from a noisy and often interfering complex stimulus environment. Secondly, the central auditory system supervises changes to the echo and rapidly evaluates feedback information,which enables the bat to adjust emitted pulse parameters instantly.Thirdly, to analyze its prey accurately, the central auditory system has accurate frequency analysis mechanisms that can handle frequency shifts caused by Doppler shifts during flight. Lastly, the central auditory system not only compares the differences between pulse and echo stimuli within the same harmonic, but also shows combination‑sensitive responses to pulse‑echo pairs in different harmonics to accurately acquire the information of relative speed and distance between the bat and its target.
1.1 Species-specific acoustic signal recognition in echolocating bats
The central auditory system can recognize species‑specific sound, which is significant for identifying different sounds generated by different bat species or by the same species, distinguishing gender,and exchanging information[8‑10]. Studies have found that AC[9,11], medial geniculate body (MGB) in the thalamus[12‑13], inferior colliculus (IC)[14‑15], lateral lemniscus[16]and other central auditory system structures are able to respond to species‑specific vocalizations, which can be affected by the neuronal modulator, serotoni[14]. Echolocating bats are also social animals that vocalize frequently to communicate with each other. But unlike other animals, species‑specific vocalization of bats is not only used for individual communication,but also used in space orientation, environment and target location,so they are usually classified as social calls and echolocation calls, respectively. It is generally believed that the social calls mainly plays an important role in the identification, communication,warning,distress and courtship between individuals in the community, while the echolocation sound plays a role in navigation and predation[17]. However,numerous studies have shown that echolocation calls serve a similar function to social calls[18‑19]. Social calls generally have a more complex spectral structure and lower frequencies than echolocation pulses which may explain these[20‑23]. In addition, the researchers studied the similarities and differences between the two calls processed by the central auditory system in bats and found different echolocation and communication feedback mechanisms, as well as the combined sensitivity of the central auditory system to social calls similar to echolocation calls[24‑25]. All in all, we can see that the two calls work together to ensure that bats are better adapted to living environment.
This article focuses on the echolocation calls,there are three main types of bats based on the spectral characteristics of their echolocation pulses:frequency modulated (FM) bats, constant frequency‑frequency modulated (CF‑FM) bats, and click bats.The acoustic signal of an FM bat has a short duration and wideband FM acoustic signal. In contrast, a CF‑FM bat’s acoustic signal is much more complex that typically includes a long CF component, usually 10-100 ms, and a narrowband FM component located at one end or both ends of the CF component[26‑27]. This sound structure enables CF‑FM bats to react specifically to both FM and CF acoustic signals.And the click bats emit faint and very brief signals of submillisecond durations. The ultra‑brief sounds consist of several shallowly FM harmonics that add up to a click‑like, broadband signal covering a frequency band of up to 80-100 kHz[26]. In addition,there is a special kind of bats called quasi‑CF bat,whose pulse is hard to be attributed to the above three kinds. The pulse pattern of quasi‑CF bat is similar to FM bats in the beginning,but a CF like signal of short duration (9-11 ms) in the terminal. Some researchers believe quasi‑CF bats are FM bats[28‑29], while the others attribute them into CF‑FM bats[30].So the quasi‑CF bats have not been included in the three main kinds yet. However, current neurophysiological study in echolocation mainly focus on FM bats and CF‑FM bats. The click bats is mainly used in the study of spatial navigation. And for quasi‑CF bats,neurophysiology studies are lack of concern except research about Japanese house bats, Pipistrellus abramu[28,31‑32].
Neurophysiological study shows that FM bats and CF‑FM bats are different not only in their pulse sound patterns but also in their neural representation in their ACs. The AC of FM bats has a topological structure organized according to frequency. Neurons tuned to high frequencies are located rostrally,whereas neurons tuned to low frequencies are located more caudally[33]. The frequency range of the FM echolocation signals is usually expanded on the cortical surface of FM bats, and the overrepresentation for FM range is related to their feeding habitat. For example, in the two frugivorous species Carollia perspicillata and Phyllostomus discolor, the FM range (60-90 kHz and 40-80 kHz respectively) is overrepresented both in the ventral tonotopic fields and in the dorsal cortex, where tonotopy is absent but neurons still exhibit a well‑tuned pure tone response. While in insectivorous bats like big brown bat, Eptesicus fuscus, the cortical overrepresentation is at 25-40 kHz, and in Molossus molossus at 30-40 kHz. Such special frequency sensitivity might represent an adaptation to the echolocation signal of FM‑bats[34]. In addition, delay‑tuned FM‑FM neurons, which have found topographically organized in the AC of CF‑FM bats,have also been found in the frontal non‑tonotopic field of Carollia perspicillata, a FM bat mentioned above.This may reveal the evolution relationship between FM bats and CF‑FM bats to some extent[35]. Unlike FM bats, the AC of CF‑FM bats can be divided into 4 specialized areas (Figure 1), respectively called Doppler‑shifted constant frequency processing area(DSCF), CF‑CF area, FM‑FM area, and dorsal fringe area (DF)[36‑39]. The DSCF region specializes mainly in processing Doppler‑shifted constant frequency component in the second harmonic. Neurons within this area are precisely tuned to frequencies to which bats are most sensitive, and their frequency tuning curves (FTCs) are extremely narrow. CF‑CF neurons play an important role in analyzing Doppler‑shifted echo and acquiring velocity information of prey.These neurons are tuned to a combination of 2-3 different CF components.Neurons in the FM‑FM area are tuned to a combination of 2-4 different FM components and can provide a precise calculation of the distance between the bat and the target.As for the DF area, it is theorized that this area may be involved in integrating information about target speed and distance as the result of converging inputs from CF‑CF and FM‑FM areas[36]. Thus, for CF‑FM bats, the FM signal is far more essential for recognition among species. But the CF‑FM pulse signal, besides being used for individual identification, is more important for a variety of echolocation tasks. Besides the topological difference between FM bats and CF‑FM bats, which is mentioned above, current studies also have comprehensively compared the differences in the auditory computation among FM bats, CF‑FM bats and other mammals. On one hand, among CF‑FM bats, researchers suggest that although mustached bats, Pteronotus parnellii, greater horseshoe bats,
Rhinolophus ferrumequinum and rufuous horseshoe bats, Rhinolophus rouxi have similarity in tonotopic primary AC, the arrangement of the anterior dorsol in the AC of rufuous horseshoe bats similar to non‑echolocating animals is different from the other two species[34]. In addition, among these CF‑FM bats,some researchers suggest mustached bat as representation because simple and complex feature detectors at every level of its auditory system are specialized to process such CF and FM components in parallel. Studies of such feature detectors led to the discovery of neuroacoustic phenomena in this species prior to their documentation in other mammals[40]. On the other hand, research shows that there is no significant difference between bats and mammals in cortical area arrangement and cortical frequency processing, but bats have special structure in echo delay time‑sensitive dorsal cortex regions, which is used for precise time perception in bats. Different bat species have either a unique chronotopic cortex topography or a distributed salt‑and‑pepper representation of echo delay[34].
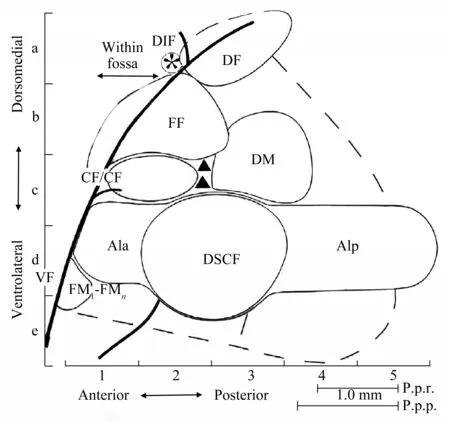
Fig.1 The functional organization of cortical auditory area in CF-FM bat[41]DSCF(Doppler‑shifted CF processing),the largest region of frequency representation, mainly processes the constant frequency component of the second harmonic.The CF/CF area contains two types neurons(CF1‑CF2 and CF1‑CF3 neurons),which analyze velocity information of prey.The FF area(FM‑FM)and DF area(dorsal fringe area)contain 3 types of neurons (FM1‑FM2, FM1‑FM3, and FM1‑FM4 neurons) that may be involved in distance calculation.
However, in IC, a crucial subcortical nucleus,where ascending information and descending signal are integrated, similar parcellation has not been determined. Three divisions of the central nucleus have different frequency representation and organized topographically, while delay tuning neurons are not topographically organized in IC, but in superior auditory nuclei[42‑43]. Besides, some studies suggest that compared to AC, IC may have higher temporal precision and collicular neurons are best suited for a fast and accurate response that could lead to fast behavioral reactions. Such results suggest a better selectivity in delay‑tuning neurons of IC than of AC[44‑45]. To further study the function of IC, our laboratory used extracellular recording methods in two kinds of CF‑FM bats (Hipposideros armiger and Hipposideros pratti) to observe the species‑specific response of IC neurons stimulated by different types of acoustic signals, like CF, FM and CF‑FM signals.We found that unlike CF or FM sounds that elicit only a single‑on (SO) response pattern in the IC, CF‑FM sounds can produce not only SO, but also double‑on(DO)response patterns in IC neurons(Figure 2)[3,46‑48].In addition, experimental results show that the latency and recovery cycle of DO neurons were significantly shorter than SO neurons, suggesting that two types of neurons may play different roles in the process of bat echolocation. Our recent research also suggests that CF‑FM sound signals can sharpen FTCs of IC neurons better than CF sound[49]. These results show that CF‑FM bat auditory neurons have a strong preference and processing capacity for species‑specific CF‑FM sound signals in IC. But the mechanism of selective preference in CF‑FM sound is unclear and needs to be further studied with intracellular recording.
In conclusion, it has been proved that most specific areas in central auditory system are used to deal with species‑specific sound signal, which is important in bats echolocation.
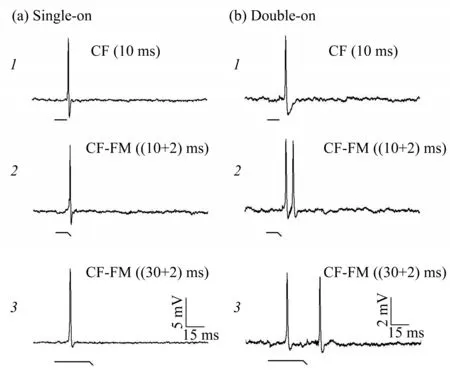
Fig.2 The responses of SO and DO neurons were recorded intracellularly in different acoustic stimulation conditions(a) 1-3, response of a SO neuron; (b) 1-3, response of a DO neuron.Note that both SO and DO neurons respond to a CF sound, but when stimulated with CF‑FM sound, only DO neurons can respond to CF and FM components of CF‑FM sounds, and when the duration of CF extended,the reaction to FM will be delayed.
1.2 Co-varying parameters processing in CFFM bats auditory system
Besides echolocating with species‑specific signals while in flight,bats also need to adjust their pulse parameters such as amplitude, duration,frequency and repetition rate since, when bats fly toward objects, the echoes produced from objects will change. For example, echo delay will decrease,intensity will increase and frequency of the echo will have a positive shift caused by the Doppler effect.All these changes force bats to monitor these stimulus features to adjust the acoustic parameters of its pulse[5,50].For example,they may shorten the duration of their pulse to avoid overlapping FM sound signals or overlapping the FM component of the CF‑FM signal(Figure 3).Other examples might be to increase the pulse repetition rate to get more environmental information within a limited time or they might reduce the frequency and intensity of the pulse to assure the frequency and intensity of echo are maintained in bat’s desired range. These parameters will change simultaneously when bats are close to the target, and thus are also known as “co‑varying parameters”[51‑52].
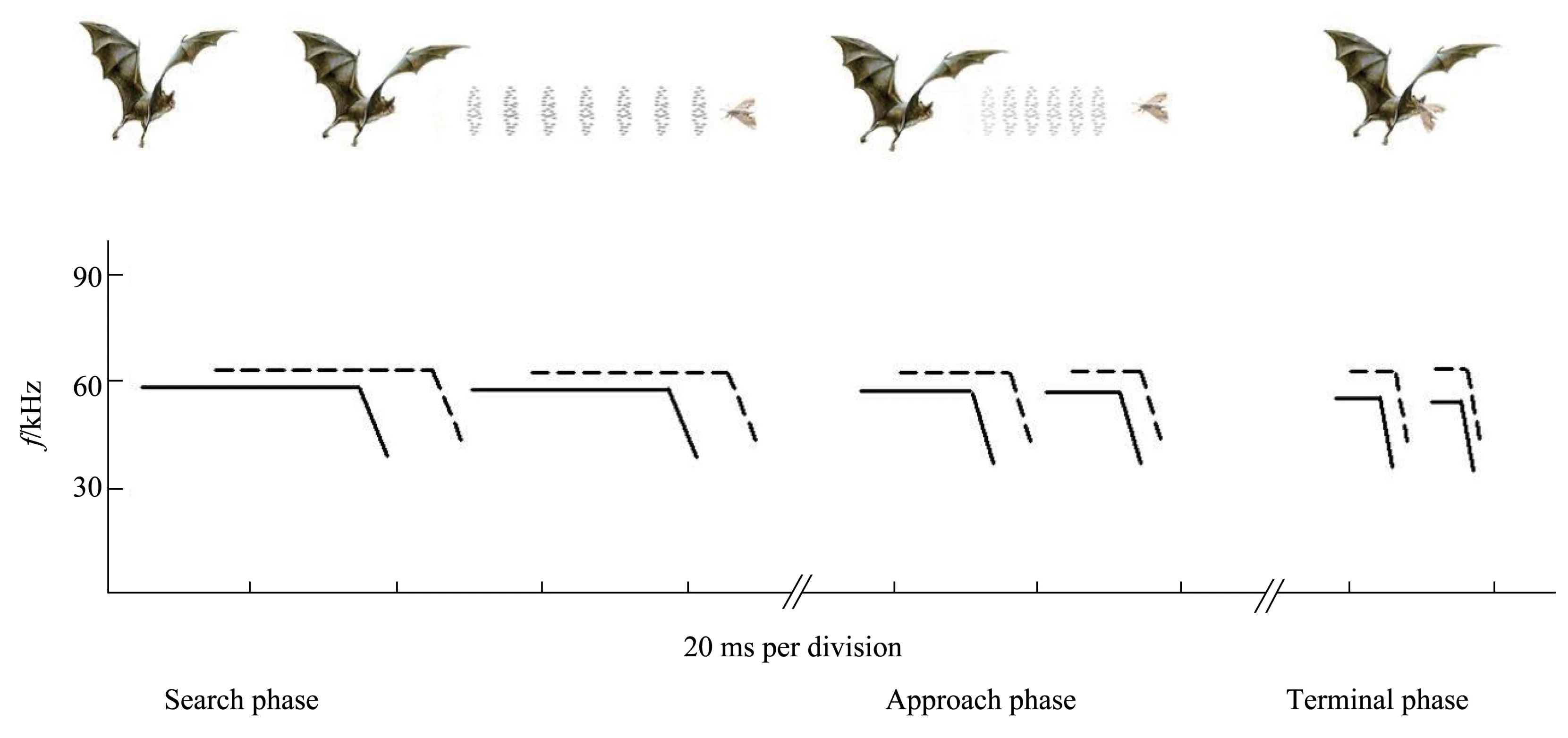
Fig.3 A mode pattern of CF-FM bat processing sound by adjusting its parameters in different phases of predation,including search phase,approach phase and terminal phaseWith the distance between bat and prey shortened,the duration of pulse shortens and the frequency of pulse is reduced.Solid lines represent the pulse,dashed lines represent the echo.The length of lines represents the duration of sound.
Research has shown that a small change in one of co‑varying parameters inevitably affects other co‑varying parameters. But how the auditory system of bats handles these co‑varying parameters has not yet been reported.The current researches include how duration, delay, amplitude, sweep rate and many other co‑variance parameters interact with each other[51,53‑57].As for FM bat, the study found that in lightly anesthetized big brown bats, the FTC of the duration‑tuned IC neurons is much narrower at its best duration(BD) than at non‑optimal durations of stimulation,and the shorter the BD, the narrower the FTC. The study also found that the bandwidth of FTC decreases as the duration and intervals of pulse‑echo pairs shorten[51,54]. It was also revealed in the awake big brown bat a correlation between duration tuning and frequency tuning in IC neurons. It seems that there is a “frequency‑duration processing trade agreement”.In general, neurons whose FTCs are narrow and sharp, the ability to process duration information is quite lacking, and vice versa[54]. In addition, this research also found that for most IC neurons,amplitude selectivity is affected by sound duration.As sound duration decreases, selectivity for intensity increases[56], and as pulse repetition rate increases,selectivity for duration enhances[57]. And for CF‑FM bats, the relationship between delay tuning and other co‑varying parameters are comprehensively investigated. Studies suggest that the activation neurons in response to delay may be regulated by pulse amplitude. It has been proved that the delay preference co‑vary with the pulse amplitude in the AC of Pteronotus parnellii, different areas that preferred to long or short delay activated differently under high pulse (80-90 dB) and fainter pulse level (60-70 dB SPL), respectively[58]. Similar result was also found that neurons tuned to short target distances are maximally responsive to low pulse amplitude while neurons tuned to long target distances respond maximally to high pulse amplitude[59]. Research also found that in the IC of Pteronotus parnellii, neurons tuned to delay also tune to sound duration[60]. What need to be attention is that sweep rate is a special parameter between FM and CF‑FM bats. It was reported that the best duration co‑vary with the same FM rate at each bandwidth in most auditory duration‑tuned neurons in the IC of big brown bat, which suggested that sweep rate of FM component was the dominant parameter for temporal encoding in FM bats[61‑62]. However, unlike FM bats, sweep rate change slightly in the preying of CF‑FM bats.According to this difference, it is predicted that it is the duration of CF component not FM sweep rate that dominant temporal tuning[59‑63]. In summary, the results above show that there may exist an adjustable matching mechanism between co‑varying parameters when facing different or rapidly changing echolocation tasks. More specifically, a bat prefers to analysis a particular parameter and coordinate other co‑varying parameters so that all parameters are matched to ensure the bat can accurately judge the target. Unfortunately, the mechanism by which the central auditory system of bat coordinates these parameters still remains unknown and needs further research.
However, we can still come to a conclusion that there are some central auditory neurons sensitive to two or more acoustic parameters and can coordinate the changes of these parameters. Such adaption may help bats response swiftly to the dynamic echo signal in different flight phase.
1.3 Auditory system function in analyzing frequency
Among the acoustic signal parameters that need adjustment in CF‑FM bats,frequency regulation is the strictest.Due to Doppler shift,a physical phenomenon in which echo frequency drifts as the result of the relative velocity between bat and target, bats need to accurately compensate their vocal frequency by increasing or decreasing the frequency of the emitted sound. This enables the bat to ensure the echo frequency is maintained in the most sensitive frequency bands during preying. Early behavioral studies reported that CF‑FM bats, including
Rhinolophidae, Hipposiderinae and Mormoopidae,have Doppler‑shift compensatory (DSC) behavior that is very accurate[24,64‑66].This accuracy is related to the auditory system of CF‑FM bats which has a special characterization area to analyze the CF component of the second harmonic of the echo. Physiological and anatomical evidence shows that the cochlea of the CF‑FM bat has a distinct special area,called the “auditory fovea”[67], which is extremely sensitive to the CF component of the second harmonic that has the highest energy in the echolocation signal[68‑69].Additionally, this property persists in upstream auditory station[70]. Similar functional areas are found in the DSCF area in the AC, both of which are considered an extension of the “acoustic fovea”. The study by Covey et al.[71]and Suga[36]also found that both in the “auditory fovea” and DSCF area, the FTCs of neurons are extremely narrow, and their capability for frequency discrimination is extremely high. For this reason, a tiny shift of the echo frequency will be instantly perceived by bats, and a bat will maintain the echo on a constant frequency through the precise DSC behavior. And to maintain the echo frequency, precise vocal control is necessary.Study have found that audio‑vocal (AV) is regulated by the neural oscillations in the fronto‑striatal network,but we do not know how the nervous system interact the AV and echolocation[72]. To explore the probable neural basis of the DSC behavior,Metzner[73‑74]studied the roles of neurons of Rhinolophus midbrain paralemniscal tegmentum in the interaction of AV,and found that all neurons in the region exhibit spontaneous discharging. These neurons can be divided into two groups with 50% of neurons belonging to the vocal inhibition units(VOC), that is, neurons with spontaneous activity that is suppressed by sound throughout the duration of the stimulus event. The rest of the neurons are vocal excitatory neurons. A vocalization enhances the excitability of spontaneous activity of these neurons,but the extent of enhancement is affected by the frequency and duration of the emitted sound. Thus,we have reason to speculate that during DSC of a CF‑FM bat, the emitted frequency shift will affect the auditory neurons to analyze the echo frequency, and as this analysis occurs, perhaps the frequency tuning of neurons becomes sharper, thereby improving the accuracy of frequency analysis. However, all of these assumptions need further studies to confirm.
Special frequency analyzing areas like “auditory fovea” and DSCF area greatly improve the accuracy of frequency analyzing, which can help bats finish complicated and accurate tasks effectively.
1.4 Auditory system function in processing multi-harmonic signals in echolocation
Any analysis of complex sounds depends on comparison of information among different spectral and temporal elements of these sounds. Research has shown that the CF‑FM bat emits bio‑pulses containing 3-4 harmonics. Each harmonic is composed of CF and FM components, and harmonics in different frequency bands are called CF1‑FM1,CF2‑FM2, CF3‑FM3and CF4‑FM4, respectively. This means a bat must distinguish different signal components from different harmonics of different bands.
But how do central auditory neurons compare this complex sound signal with different harmonics?Research shows that combination‑sensitive neurons play a major role in comparing information with different spectral‑temporal sound features. These neurons have been found in IC, MGB and AC[75‑79].The primary type of these neurons which are called combination‑sensitive facilitated neurons shows a larger facilitative response to a combination of multiple signal elements than the algebraic sum of responses to individual signal elements. For example,a combination‑sensitive response can be induced in this type of neuron when the emitted pulse is FM or CF signal of first harmonic and the delayed echo is FM or CF signal of a higher harmonic(FMn/CFn,n=2,3 or 4). These neurons have important biological significance in achieving effective prey information.For example, an early study of the AC of the mustache bat found that combination‑sensitive neurons can be divided into two categories: CF‑CF neurons and FM‑FM neurons, the former mainly analyzes the relative velocity of the target, whereas the latter mainly encodes distance information.
In recent years, many pharmacological and electrophysiological studies have discussed the mechanism underlying that combination‑sensitive neurons integrating spectro‑temporal information[45,80‑83]. Many of these findings suggest the IC plays a key role in producing combination‑sensitive facilitation response. To a great extent, this response is dependent on timely glycine inhibition followed by rebound excitation, that is, IC neurons can receive glycinergic input from the low frequency and high frequency projection areas[84]. However,Peterson et al.[85]used intracellular recording to determine whether IC neurons accept the low‑frequency inhibitory inputs, and found that most FM‑FM combination‑sensitive neurons of the mustache bat IC do not exhibit inhibitory postsynaptic potentials and excitatory rebound evoked by low frequency.
In other mammals like mouse, cat, guinea pig,ferret, mongollan gerbli and macaque monkey, the mechanism of combination‑sensitive may be different from bats. Because in bats, echo delay time‑sensitive dorsal cortex regions contain special designs which is more powerful in time perception. Such difference suggests that combination‑sensitive could be faint or even absent in other mammals[34]. Therefore, it is imperative that the neural mechanism of combination‑sensitive among different species is explored with intracellular recording technique and neuropharmacology research methods.
Although the mechanism of combination‑sensitive may be different among species, above studies suggest that combination‑sensitive help dealing multi‑harmonic signal in bats, which is necessary in distance measure in echolocation.
2 Expectation
This review is mainly about the neural mechanism underlying that CF‑FM bats process the sound signal related to echolocation behavior, which will not only help us understand the strategy of how the bat auditory system processes echolocation signals and its neural mechanisms, but also to raise some issues that remained unresolved.
This review also uses CF‑FM bats as a model to conclude the behavioral correlation of acoustic signal processing and found that there are some speciality.Behavioral studies have proved that CF components have a distinct role in rejection of clutter and mitigation of ambiguity, which is beneficial in cluttered environments that CF‑FM bats live[86].Neurophysiological studies have also found that,besides areas dealing with FM signal, CF‑FM bats also have specialized areas for CF signal. Such as auditory fovea in cochlea, which is extremely sensitive to the CF‑component of second harmonic.This specialized structure persists in upstream auditory centers, and in AC is called DSCF area,which also imply there is a special circuit that support DSC[37]. But whether such property is applicable for other vocalizing mammals worth further exploration.What’s more,many CF‑FM bats,like mustached bats,have been well studied yet. Discovery of neuroacoustic phenomena in this species prior to their documentation in other mammals. For instances,include delay tuning, spectral and temporal combination‑sensitivity[87‑89]and disproportionate tonotopic representation[90], So here we suggest that taking CF‑FM bats as model may be beneficial under some circumstances.

