Study on the in vitro anti ovarian cancer effect and mechanism of quinazoline derivative (N111)
LI Yan, HUANG Qiang, HUANG Yin-jiu✉, LIU Gang, LIU Jian✉
1.Department of Gynecology and Oncology, the First Affiliated Hospital of Bengbu Medical College, Bengbu 233099, China
2.College of Life Sciences, Bengbu Medical College, Bengbu 233030, China;
3.School of Chemistry and Materials Science, Ludong University, Yantai 264025, China
Keywords:
ABSTRACT Objective: To study the anti-ovarian cancer effect and mechanism of Quinazoline derivative(N111) in vitro; Method: Using an online database to predict the therapeutic targets of N111 for ovarian cancer, and conducting biological functional analysis of the therapeutic targets.The experiment was divided into N111 treatment group (N111 compound group), positive control group (cisplatin group), and negative control group (DMSO group); After grouping,MTT assay was used to detect cell proliferation; Morphological observation was used to observe changes in cell morphology; JC-1 and DCFH-DA probes were used to detect the changes of mitochondrial Membrane potential and intracellular reactive oxygen species; PI,Annexin V-FITC, and DAPI staining were used to detect cell cycle arrest and apoptosis; Clone formation experiments and scratch tests were conducted to detect the cell's ability to form clones and migrate; Western blot method was used to detect the expression level of related proteins.Result: The biological function research results show that the biological function of N111 anti ovarian cancer target protein suggests that the target function aggregates human diseases, inflammation, tumors, and other aspects.Compared with the control group, N111 has a significant inhibitory effect on the proliferation of ovarian cancer cells (IC50=14.62 mmol/L) (P<0.0001); In a concentration dependent manner, it inhibited the formation and migration of single cell colonies, and induced the disorder of mitochondrial Membrane potential, ROS and cell cycle arrest in S phase (P<0.0001); As the concentration of N111 treatment increased,the expression levels of Bcl2, Caspase 3, P-AKT, and SHIP2 decreased, while the expression levels of AKT remained unchanged.The expression levels of Bax and Cleared Caspase 3 increased (P<0.0001).Conclusion: Compound N111 inhibits SHIP2, promotes ROS level disorder, weakens the activation of AKT signaling pathway, and thus inhibits the proliferation,migration, and clone formation of tumor cell A2780, inducing cell apoptosis.
1.Introduction
Ovarian cancer (OC) is one of the most common causes of death in gynecological cancer[1,2].The use of anti-tumor drugs can induce cancer cell stress and promote tumor cell apoptosis, as well as induce excessive production of reactive oxygen species, leading to oxidative stress and ultimately serious damage to the body.Therefore, the application of antioxidants in anti-cancer is worth further research[3].
Quinazoline is the most widely used nitrogen containing Heterocyclic compound.The compounds with Quinazoline Pharmacophore have been widely used in the treatment of tumors[4-7].In Drug development, metal elements are often incorporated into bioactive ligands, increasing the biological activity of ligands and enhancing the pharmacological potential of inactive ligands[8,9].Selenium is a naturally occurring trace non-metallic element with antiviral and antioxidant properties.Research has shown that selenium can be used to prevent and treat various diseases and tumors[10,11].Studies have shown that Selenium compounds can enhance insulin sensitivity and prevent inflammation and oxidative stress by inhibiting phosphatidylinositol-5-phosphatase (SHIP2)[12].Compounds containing selenium and Quinazoline Pharmacophore can induce cell apoptosis through PI3K/AKT signaling pathway and inhibit cell autophagy[13].The Pharmacophore connecting Quinazoline derivatives and phenolic derivatives has strong antioxidant properties due to the addition of effects[14].However, no research on selenium containing Quinazoline derivatives in ovarian cancer has been found.
Therefore, this study synthesized a new selenium containing Quinazoline derivative based on two compounds with anti-cancer properties.Through MTT screening, N111 has significant anti-tumor properties.Through bioinformatics, the functional analysis results of N111 anti ovarian cancer targets were verified and predicted with the anti-tumor action and mechanism of N111 in vitro, and further explored the anti-tumor mechanism of N111 on ovarian cancer.
2.Materials
Laboratory synthesis of 2-ethoxy-4-ethylselenyl Quinazoline(N111) belongs to selenium containing Quinazoline compounds and organic selenium compounds (Annex 1).Use Dimethyl sulfoxide(DMSO) to prepare 40 mmol/L stock solution.Cells originate from the laboratory.Cycle, apoptosis, reactive oxygen species (ROS)detection kit purchased from Biyuntian Biotechnology Company.Antibodies: β- Actin, apoptotic protein (Bax), anti-apoptotic protein(Bcl2), Caspase-3, Cleared Caspase-3 were purchased from Wuhan Sanying Biotechnology Company, and Protein kinase B (AKT),SHIP2 were purchased from Albotec Biotechnology Company.All other chemical reagents are analytical reagent grade.The human ovarian cancer cell lines A2780, HEY, ES-2, and human normal ovarian epithelial cell line IOSE80 were all sponsored by our laboratory’s senior sister and classmates.
3.Methods
3.1 Drug structure and target acquisition
Analyze the selected compound structural formula through online websites and predict drug treatment target information based on the compound structure.(http://www.swisstargetprediction.ch, https://www.genecards.org/, https://go.drugbank.com/, https://omim.org/)[15].
3.2 molecular docking
Prepare 3D formats for small molecule ligands and large molecule receptors, convert PDBQT format using AutoDock Tools 1.5.7 software, and establish receptor protein activity pockets.Perl script performs Macromolecular docking analysis.Present a virtual docking model between target proteins and compounds using PyMOL2.5.1 software.
3.3 Cell culture and passage
Prepare fresh RPMI-1640 complete culture medium according to the proportion requirement of complete culture medium (basic culture medium: serum=9:1).The cells were cultured in the incubator at 37 ℃ and 5% CO2humid environment with the culture medium (1640) containing 10% Fetal bovine serum (FBS) and penicillin Streptomycin.Daily observation of cell growth status and cell density allows for cell passage, with a density of 75% to 80%.Generally, fluid exchange or passage procedures are performed every 2-3 d.
3.4 Morphological observation method
A 96 well plate with a density of 4×103/well and incubate routinely in an incubator.The next day, the concentration of the prepared drug was (0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L.40 mmol/L) Incubate the working solution with the selected compound at different concentrations for 24 h, and observe the morphological changes of the cells under an inverted microscope.
3.5 MTT colorimetric method
A 96 well plate with a density of 4×103/well and incubate routinely in an incubator.The next day, adjust the concentration of the compound with an appropriate amount of complete culture medium,and prepare the drug with a concentration of (0 mmol/L.5 mmol/L.10 mmol/L.15 mmol/L.20 mmol/L.25 mmol/L.30 mmol/L.35 mmol/L.40 mmol/L) Incubate the working solution with the selected compound at different concentrations for 24 h, with 3 secondary wells in each group, and incubate it in an incubator for routine cultivation.After 24 h, add 10 μL MTT working solution to each well in an incubator for 4 h.After 4 h, use DMSO (100 μL/well Dissolve formaldehyde and measure the optical density value using an enzyme-linked immunosorbent assay (570 nm).
3.6 Cell cycle analysis
Cells were inoculated in a 6-well plate and routinely cultured in an incubator.The next day, at different concentrations (0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L) Incubate for 24 h.After 24 h,pancreatic enzymes were collected and cells were resuspended using pre cooled 75% ethanol, and stored overnight at 4 ℃.The next day,according to the instructions of the reagent kit, prepare PI staining working solution, incubate for 30 min, and perform flow cytometry on the machine for detection.
3.7 Detection of intracellular reactive oxygen species (ROS)
Cells were inoculated in a 6-well plate and routinely cultured in an incubator.The next day, at different concentrations (0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L) Incubate for 24 h.According to the instructions of the reagent kit, prepare the staining working solution, incubate for 30 min, and detect it by flow cytometry.
3.8 DAPI
Cells were inoculated in a 6-well plate and routinely cultured in an incubator.The next day, at different concentrations (0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L) Incubate for 24 h.After 24 h, remove the board, clean it twice with PBS, and use it at room temperature for 200 h μL 4% Paraformaldehyde fixation for 30 min, 200 μL 0.1% TritonX-100/PBS was transparent for 30 min,DAPI staining was performed for 5 min, and an appropriate amount of PBS was used to moisten the cells.Photos were taken under a fluorescence microscope.
3.9 Determination of mitochondrial Membrane potential
Cells were inoculated in a 6-well plate and routinely cultured in an incubator.The next day, at different concentrations (0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L) Incubate for 24 h.Collect cells,prepare JC-1 staining solution according to the instructions of the kit, incubate for 20 min, detect with flow cytometry, and take photos under an inverted microscope.
3.10 Cell apoptosis detection
Cells were inoculated in a 6-well plate and routinely cultured in an incubator.The next day, at different concentrations (0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L) Incubate for 24 h.According to the instructions of the reagent kit, prepare the staining working solution, incubate for 30 min, and detect it by flow cytometry.
3.11 Scratch experiment
Cells were inoculated in a 6-well plate and routinely cultured in an incubator.The next day, at different concentrations (0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L) Incubate for 24 h.The next day,use a 200 μL yellow gun to vertically scrape scratches.Clean the cell fragments with PBS, and immediately observe and record under an inverted microscope (at this time, the scratch area is 0 h).After recording, place them back in the incubator and continue regular cultivation for 24 h.After 24 h, take out the plate, clean it twice with PBS, add an appropriate amount of low serum culture medium, and immediately observe and take photos under an inverted microscope(this is the 24 h scratch area).Use Image J to calculate the scratch area value.24 h migration rate (%) = (scratch area in 0 h - scratch area in 24 h)/scratch area in 0 h) × 100%.
3.12 colony forming assay
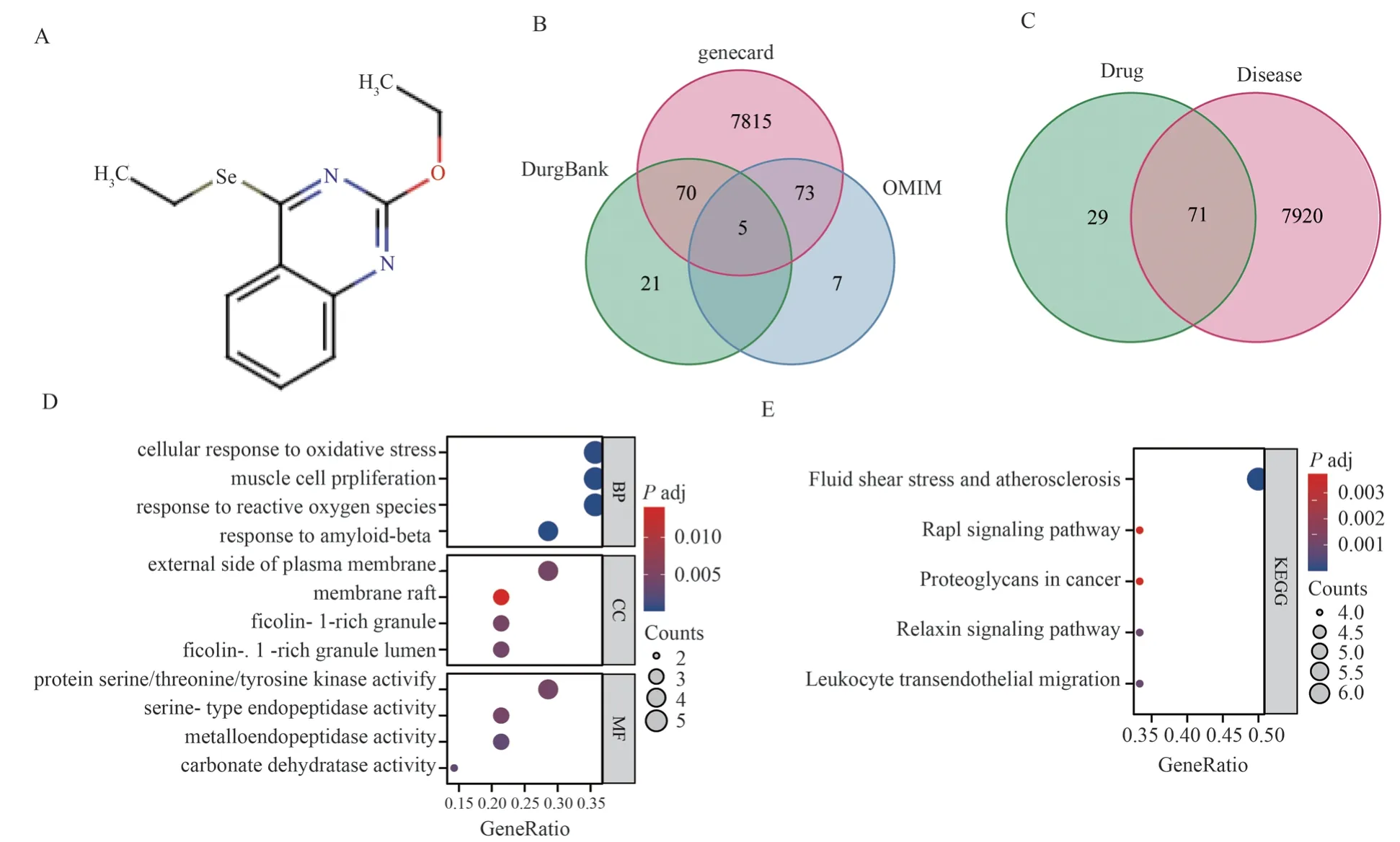
Fig 1 Functional analysis of N111 target and ovarian cancer treatment target: (A) drug structure of N111 (B) Vene diagram showing ovarian cancer treatment target (C) Vene diagram showing disease and drug common target (D) GO analysis of common target (E) KEGG analysis of common target
Cells were inoculated in a 6-well plate (500 cells/well) and routinely cultured in an incubator.The next day, at different concentrations(0 mmol/L.5 mmol/L.10 mmol/L.20 mmol/L) Incubate for 24 h.After 2 d, replace the fresh complete culture medium to continue the routine culture.Observe the size of cell clusters or wait for 14 dafter the routine culture.PBS is washed twice, fixed with methanol for 20 min, stained with 0.1% Crystal violet for 30 min, photographed with mobile phones, and calculated by Image J.
3.13 Western blot detection Bax、Bcl2、Caspase 3、Cleaved Caspase-3、AKT、P-AKT、SHIP2
Cells were inoculated in a 6-well plate and incubated with the selected compound at different concentrations for 24 h.RIPA lysis buffer was used to freeze and lyse cells for 30 min, and 12 000 r/min was centrifuged for 15 min.Collect the supernatant and measure the protein concentration using BCA method.12.5% SDS polyacrylamide gel was used to isolate protein (30 μg/well), Transfer to 0.45 μM sized polyvinylidene fluoride film.Seal the membrane with 5% skimmed milk at room temperature [prepared with Tris Buffed saline (TBS) containing 0.1% Tween] for 2 h, and incubate the primary antibody overnight in a refrigerator at 4 ℃.Incubate with the secondary antibody at room temperature for 2 h, measure the signal using an enhanced chemiluminescence detection kit, and visualize it using the ChemiDocTM imaging system.To resist β-Actin serves as an internal reference control.
3.14 Statistical processing
Using GraphPad Prism 5 software to analyze data, t-test was used for inter group analysis of measurement data, and analysis of variance was used for 3 or 4 group analysis.The difference was statistically significant according to P<0.05.
4.Results
4.1 Functional analysis of N111 targets and ovarian cancer treatment targets
According to the chemical structural formula of N111,the drug structural formula of N111 was displayed on the Swisstargetprediction online website (Figure 1A), and the top 100 target proteins predicted for N111 anti ovarian cancer were obtained through the SwisstargetPrediction database.Vene plot analysis revealed a total of 7991 treatment targets for ovarian cancer in Genecard, DrugBank, and OMIM databases (Figure 1B).Vene plot obtained 7991 treatment targets for ovarian cancer and 71 common target proteins predicted by N111 for anti ovarian cancer treatment targets (Figure 1C).GO functional analysis was conducted on 71 common target proteins, and the results showed that the target proteins were mainly enriched in hypoxia response, angiogenesis,membrane structure, signal pathway protein activation, etc.(Figure1D), while the biological function KEGG was concentrated in human diseases, inflammation, tumors, etc.(Figure 1E).
Tab 1 Toxic effects of N111 on ovarian cancer cell line A2780 (n=3,±s)

Tab 1 Toxic effects of N111 on ovarian cancer cell line A2780 (n=3,±s)
Note: with a concentration of 0 μ Mol/L ratio, * P < 0.05** P < 0.01*** P < 0.001**** P<0.000 1.
Concentrations of N111(mmol/L) IOSE80 A2780 Concentrations of DDP(mmol/L) IOSE802 A27803 0 0.29±0.01 0.29±0.01 0 0.26±0.00**** 0.35±0.01 0.28±0.01* 0.28±0.01* 2 0.24±0.00**** 0.34±0.00 5 2 0.26±0.00** 0.26±0.01**** 5 0.23±0.00**** 0.30±0.00***10 0.26±0.00**** 0.26±0.00**** 10 0.23±0.00**** 0.26±0.02****15 0.24±0.01**** 0.24±0.00**** 15 0.22±0.00**** 0.25±0.01****20 0.22±0.01**** 0.22±0.01**** 20 0.22±0.00**** 0.22±0.01****25 0.21±0.00**** 0.19±0.00**** 25 0.2±0.00**** 0.21±0.00****30 0.20±0.00**** 0.18±0.00**** 30 0.17±0.00**** 0.18±0.01****35 0.19±0.00**** 0.17±0.00**** 35 0.13±0.00**** 0.16±0.00****40 0.16±0.00**** 0.16±0.00**** 40 0.12±0.00**** 0.15±0.01****F 79.21 197.7 820.5 102.1 P<0.000 1 <0.000 1 <0.000 1 <0.000 1

Fig 2 Molecular simulation docking of receptor proteins SHIP2, PIK3CA and AKT1 with ligand N111
4.2 Macromolecular docking
The SHIP2, PI3KCA and AKT1 were respectively docked with N111 by molecular simulation, and the Macromolecular docking energy models of -4.2, -6.1 and -4.3 (kcal/moL) were selected for display (Figure 2).
4.3 N111 inhibits the growth and proliferation of ovarian cancer cells
After 24 h of exposure to different concentrations of N111, cell morphology was observed to induce cell deformation, atrophy, and rupture in a concentration dependent manner (Figure 3A).With cisplatin (DDP) as the positive control, the results showed that N111 had a concentration dependent Sexual inhibition of cell proliferation(IC50=14.62 mmol/L) (P<0.0001) (Figure 3B, Table 1).Through flow cytometry analysis of cell cycle, the proportion of G1 phase cells decreased from 66.9 ± 0.86% in the control group to 5% in the experimental group after N111 treatment of A2780 cells mmol/L (55.93 ± 1.36)%, 10 mmol/L (37.43 ± 0.86)%, 20 mmol/L (21.50± 0.80)%; The proportion of S phase cells increased from (15.47 ±0.38)% in the control group to 5 in the experimental group mmol/L(21.83 ± 1.15)%, 10 mmol/L (42.10 ± 0.65)%, 20 mmol/L (58.03 ±1.03)% (P<0.0001) (Figure 3 C-D, Table 2).
Tab 2 N111 induced A2780 cell cycle arrest(n=3,±s)

Tab 2 N111 induced A2780 cell cycle arrest(n=3,±s)
Note: with a concentration of 0 μMol/L ratio, * P<0.05*** P<0.001****P<0.000 1.
concentrations (mmol/L) G1 S G2 0 66.90±0.86 15.47±0.39 17.43±0.60 5 55.93±1.36**** 21.83±1.16*** 21.17±0.65***10 37.43±0.86**** 42.10±0.65**** 17.80±0.45 20 21.50±0.80**** 58.03±1.03**** 15.60±0.43*F 811.1 1020 36.36 P<0.0001 <0.0001 <0.001
4.4 ROS activity level of N111
After 24 h of exposure to different concentrations of N111, the intracellular ROS level significantly decreased, The fluorescence intensity of the concentration group 20 mmol/L actually increased,which is due to the increased anti-tumor effect of N111 caused by excessive drug concentration, resulting in more ROS produced by cell stress and inducing cell apoptosis.Average fluorescence intensity * 103: control group (302.95 ± 15.84), 5 mmol/L (270.12± 3.68), 10 mmol/L (159.92 ± 2.05), 20 mmol/L (244.58 ± 2.49)(P<0.0001) (Figure 4, Table 3).
Tab 3 N111 induces ROS production in ovarian cancer cell line A2780 (n=3,±s)

Tab 3 N111 induces ROS production in ovarian cancer cell line A2780 (n=3,±s)
Note: with a concentration of 0 μMol/L ratio, * P<0.05*** P<0.001****P<0.000 1.
concentrations (mmol/L)(×103) images/BZ_15_1007_1880_1023_1911.png±s 0 302.95±15.84 5 270.12±3.68*10 159.92±2.05****20 244.58±2.49***F 109.1 P<0.0001
4.5 N111 induces apoptosis in ovarian cancer cells
After 24 h of treatment with different concentrations of N111 on A2780 cells, the number of cells decreased, the nucleus pyknosis and rupture occurred (Figure 5A).The detection results of the JC-1 fluorescence probe inside the cell indicate that after N111 treatment,as its concentration gradually increases, the red fluorescence inside the cell gradually weakens and the green fluorescence gradually increases (Figure 5B).The flow cytometry detection results showed that as the concentration of N111 increased, the proportion of cells in the second/third quadrant significantly decreased: the control group (10.69 ± 0.77), 5 mmol/L (9.71 ± 0.92), 10 mmol/L (5.38 ±0.16), 20 mmol/L (2.94 ± 0.08) (P<0.0001) (Figure 5C, Table 4).Flow cytometry analysis of apoptosis showed that with the increase of N111 concentration, the total apoptosis rate of cells (the total proportion of second and third quadrant cells) significantly increased.The total apoptosis rate was as follows: control group (12.07 ± 0.56),5 mmol/L (13.56 ± 0.61), 10 mmol/L (18.48 ± 0.61), 20 mmol/L(22.64 ± 0.40) (P<0.0001) (Figure 5D, Table 5).

Fig 3 N111 inhibits the proliferation of ovarian cancer cells: (A) N111 induces morphological changes in cells; (B) N111 inhibits cell proliferation; (C-D)N111 induces cell cycle arrest in S phase
4.6 N111 inhibits ovarian cancer Cell migration
After 24 h of treatment with different concentrations of N111 on A2780 cells, N111 significantly inhibited the migration ability of A2780 cells in a concentration dependent manner.The migration rates were as follows: control group (13.87 ± 1.22), 5 mmol/L(13.73 ± 0.34), 10 mmol/L (11.03 ± 0.24), 20 mmol/L (10.63 ±0.91) (P<0.0001) (Figure 6A, Table 6).N111 significantly inhibited the monoclonal formation ability of A2780 cells in a concentration dependent manner, with a clone formation rate of : in the control group :(0.87 ± 0.10) and 5 mmol/L (0.64 ± 0.03), 10 mmol/L (0.26 ±0.04), 20 mmol/L (0.06 ± 0.01) (P<0.0001) (Figure 6B, Table 7).

Fig 5 N111 induced apoptosis of ovarian cancer A2780 cells: (A) N111 induced nuclear change (B) N111 induced mitochondrial membrane potential staining change (C) N111 induced mitochondrial membrane potential change (nsP 0.05;****P<0.000 1)(D) N111 induced apoptosis(nsP 0.05;****P<0.000 1)
Tab 4 Red green fluorescence ratio of N111 induced changes in mitochondrial membrane potential of ovarian cancer cell A2780(n=3, ±s)

Tab 4 Red green fluorescence ratio of N111 induced changes in mitochondrial membrane potential of ovarian cancer cell A2780(n=3, ±s)
Note: with a concentration of 0 μ Mol/L ratio, **** P<0.000 1.
Concentrations images/BZ_15_1007_1880_1023_1911.png±s 10.69±0.77 5 9.71±0.92 0 10 5.38±0.16****20 2.94±0.08****F 71.57 P<0.0001
Tab 5 N111 induces apoptosis in ovarian cancer cell line A2780(n=3, ±s)

Tab 5 N111 induces apoptosis in ovarian cancer cell line A2780(n=3, ±s)
Note: with a concentration of 0 μMol/L ratio, **** P<0.000 1.
concentrations images/BZ_15_1007_1880_1023_1911.png±s 0 12.07±0.56 5 13.56±0.61 10 18.48±0.61****20 22.64±0.4****F 153.4 P<0.0001
Tab 6 N111 inhibits the migration of ovarian cancer cell line A2780(n=3,±s)

Tab 6 N111 inhibits the migration of ovarian cancer cell line A2780(n=3,±s)
Note: with a concentration of 0 μ Mol/L ratio, * P<0.05** P<0.01.
concentrations images/BZ_15_1007_1880_1023_1911.png±s 0 13.87±1.22 5 13.73±0.34 10 11.03±0.24*20 10.63±0.91**F 9.5 P 0.0052
Tab 7 N111 inhibits the migration of ovarian cancer cell line A2780(n=3,±s)

Tab 7 N111 inhibits the migration of ovarian cancer cell line A2780(n=3,±s)
Note: with a concentration of 0 μMol/L ratio, * P<0.05**** P<0.000 1.
Concentrations images/BZ_15_1007_1880_1023_1911.png±s 0 0.87±0.10 5 0.64±0.03*10 0.26±0.04****20 0.06±0.01****F 82.15 P<0.0001

Fig 6 N111 inhibits migration and monoclonal formation of ovarian cancer A2780 cells: (A) N111 inhibits cell migration; (B) N111 inhibits the formation of monoclonal cell clusters
4.7 N111 exerts anti ovarian cancer effects through the SHIP2/AKT signaling pathway
After 24 h of N111 treatment of A2780 ovarian cancer cells, the expression levels of Bcl2, Caspase 3, P-AKT, and SHIP2 gradually decreased, while AKT remained unchanged.The expression levels of Bax and Cleared Caspase 3 gradually increased.(Figure 7, Table 8).
5.Discussion
At present, the application of Pharmacophore hybridization principle is one of the important methods to design and develop new drug compounds, and hybrid coupling compounds have potential dual effects[16, 17].In this study, the antioxidant structure (selenium)and the anti-tumor Pharmacophore (Quinazoline) were coupled to obtain the final compound N111, and further study the anti ovarian cancer effect of N111.

Fig 7 N111 exerts anti ovarian cancer effect through SHIP2/AKT signal pathway(nsP 0.05;****P<0.000 1).
Tab 8 Changes in protein expression of ovarian cancer cell A2780 after N111 treatment(n=3,±s)

Tab 8 Changes in protein expression of ovarian cancer cell A2780 after N111 treatment(n=3,±s)
Note: with a concentration of 0 μ Mol/L ratio, * P < 0.05** P < 0.01*** P < 0.001**** P<0.000 1.
Concentrations Bax Cleaved Caspase3 Bcl2 Caspase3 Akt P-Akt SHIP2 0 0.94±0.11 0.94±0.06 0.97±0.09 0.98±0.09 0.96±0.04 0.94±0.06 0.93±0.06 1.17±0.06 1.18±0.02*** 0.85±0.09 0.81±0.05 1.10±0.08 0.73±0.05* 0.70±0.08*10 1.29±0.06** 1.22±0.03**** 0.67±0.08* 0.58±0.06*** 1.07±0.09 0.53±0.05*** 0.33±0.05****20 1.33±0.07** 1.48±0.02**** 0.43±0.07*** 0.37±0.04**** 1.10±0.08 0.30±0.08**** 0.14±0.04****F 9.596 67.7 15.71 32.83 1.49 39.28 72.19 P 0.005 <0.001 0.001 <0.001 0.29 <0.001 <0.001 5
SHIP2 is a protein encoded by the inositol polyphosphate phosphatase like 1 (INPPL1) gene.When PI3K catalyzes PI (4,5)P2 to become the second messenger PI (3,4,5) P3, SHIP2 converts it into PI (3,4) P2, which has strong affinity with the Pleckstrin homology domain of AKT [18,19].Previous studies have suggested that SHIP2 exerts an anti-tumor effect by degrading PI (3,4,5) P3,thereby inhibiting the activation of AKT.In the study of gastric cancer, by reducing the expression of SHIP2 protein, activating the PI3K-AKT signaling pathway, and promoting cell proliferation[20].However, most studies show that SHIP2 can promote Cell migration, invasion, reduce cell adhesion and other functions[21],and induce colorectal cancer cells to increase drug resistance[22].In the study of ER negative breast cancer, SHIP2 activates AKT and JNK, upregulates VIM, and increases tumorigenicity[23].The high expression of SHIP2 leads to low disease-free survival and overall survival in patients with laryngeal squamous cell carcinoma[24].Among them, in the study of laryngeal squamous cell carcinoma,downregulation of SHIP2 inhibited the activation of AKT signaling pathway, thereby inhibiting cell proliferation, promoting cell cycle arrest and apoptosis, and enhancing the radiosensitivity of cancer cells.The mechanism may be that the product of SHIP2 has a strong affinity for AKT, and inhibiting SHIP2 leads to a partial decrease in AKT activity and corresponding receptor expression[25].Another study also suggests that if the PI3K-AKT signaling pathway is stimulated by growth factors, AKT phosphorylation is enhanced.If stimulated by hydrogen peroxide, SHIP2 activity is inhibited, and AKT phosphorylation is weakened[26].Consistent with our observations, SHIP2 inhibition in colorectal cancer cells reduces AKT phosphorylation[22].Similarly, in this study, N111 suppressed cell proliferation, induced cycle arrest, and apoptosis by downregulating SHIP2 and inhibiting AKT phosphorylation.
Furthermore, the biological function analysis of N111 anti-ovarian cancer targets was conducted through a database.Similarly, the results of the database analysis were consistent with our cytological experiments, indicating that the analysis results of N111 through the database have strong reliability.According to the prediction of drug action mechanism in the database, the anti-tumor mechanism of N111 is related to the Receptor tyrosine kinase signaling pathway and oxidative stress signaling pathway.
In summary, compound N111 inhibits SHIP2, promotes ROS level disorder, weakens the activation of AKT signaling pathway, and thus inhibits the proliferation, migration, and clone formation of tumor cell A2780, inducing cell apoptosis.
 Journal of Hainan Medical College2023年19期
Journal of Hainan Medical College2023年19期
- Journal of Hainan Medical College的其它文章
- m6A modification promotes the proliferation and migration of cervical cancer and regulates the expression of PD-L1
- Expression and correlation of pyroptosis-related markers and PI3K/AKT pathway in endometriosis
- Meta-analysis of the efficacy of volar plate internal fixation versus closed reduction and external fixation in the treatment of adult distal radius fractures
- A review of the epidemic and clinical study on scrub typhus in China(2010-2020)
- MiR-15a-5p in neutrophil exosomes promotes macrophage apoptosis through targeted inhibition of BCL2L2
- Clinical efficacy of bushen huatan huoxue recipe in combination with acupuncture in treating patients suffering from polycystic ovary syndrome with insulin resistance
