m6A modification promotes the proliferation and migration of cervical cancer and regulates the expression of PD-L1
YANG Jing-rui, XIA Na-na, YU Min-min
Department of Gynaecology, Nanjing Hospital Affiliated to Nanjing University of Chinese Medicine, Nanjing 210003, China
Keywords:
ABSTRACT Objective: To explore the effects of N6-methyladenine (m6A) modification-related genes,methyltransferase 14 (METTL14), and YTH domain family protein 1 (YTHDF1), on the proliferation, migration and apoptosis capabilities of cervical cancer cells and investigate their correlation with programmed cell death-ligand 1 (PD-L1) expression.Methods: The expression levels of METTL14, YTHDF1 and PD-L1 in cervical cancer tissues and normal cervical tissues were analyzed using immunohistochemistry.Small interfering RNA (siRNA)was used to knock down the expression of METTL14 and YTHDF1 genes in cervical cancer cells, and the knockdown efficiency was validated by real-time fluorescent quantitative PCR(qPCR).After knockdown of METTL14 and YTHDF1, cell proliferation was assessed by CCK-8 assay, cell migration was examined by Transwell assay, cell apoptosis was detected by flow cytometry, and PD-L1 mRNA and protein expression were evaluated using qPCR and Western blotting, respectively.Results: Immunohistochemistry results demonstrated high expression of METTL14, YTHDF1, and PD-L1 in cervical cancer tissues.Knockdown of METTL14 and YTHDF1 significantly inhibited the proliferation and migration capabilities of cervical cancer cells, increased apoptosis, and downregulated PD-L1 mRNA and protein expression levels.Conclusion: m6A methylation modification can affect the proliferation,migration and apoptosis of cervical cancer cells by regulating the expression of PD-L1 in cervical cancer cells.
1.Introduction
Cervical cancer is the most common gynecological malignant tumor, posing a significant threat to women’s life and health.In 2020, China reported nearly 110,000 new cases and almost 60,000 deaths from cervical cancer, accounting for approximately 18.3%and 17.6% of the global incidence and mortality, respectively, and there is a trend of increasing cases among younger patients[1].The clinical practice guidelines for cervical cancer published in 2022 by the National Comprehensive Cancer Network (NCCN) in the United States stated that for advanced cervical cancer, the firstline treatment of choice is chemoradiotherapy.For patients with persistent or recurrent metastatic cervical cancer and positive PDL1(CPS≥1) or MSI-H/dMMR, the preferred first-line combination chemotherapy is the programmed death 1 (PD-1) inhibitor pembrolizumab ±cisplatin(carboplatin)±paclitaxel±bevacizum ab(evidence level 1)[2].Tumor immunotherapy has gradually become a research focus in cervical cancer treatment, however, the overall response rate of PD-1 inhibitors alone is only about 20%[3].Therefore, exploring more effective immune combination therapies and immunotherapy response biomarkers is particularly crucial for the treatment of cervical cancer.
N6-methyladenosine (m6A) modification is a gene regulatory mechanism that is closely associated with the occurrence of various cancers, including cervical cancer, endometrial cancer, and squamous cell carcinoma[4].m6A modification is widely present in eukaryotes, and research has indicated its dynamic regulation is significantly correlated with gene expression, potentially impacting the expression of PD-L1 through other pathways[5].Methyltransferase-like 3 (METTL3) mediates m6A methylation of PD-L1 mRNA in the near stop codon region, thereby regulating PD-L1 expression and maintaining gene stability[6].Additionally,an analysis of clinical and transcriptomic data from 306 cervical cancer patients in The Cancer Genome Atlas (TCGA) dataset revealed a close correlation between m6A modification patterns and immune therapy response, suggesting its critical role in cervical cancer initiation and progression[7].Currently, the precise role of m6A modification in the development of cervical cancer remains incompletely understood, and no reports have explored its association with PD-L1 expression.Therefore, this study aims to observe the expression of m6A modification-related genes and PDL1 in cervical cancer tissues, exploring their impact on cervical cancer cell proliferation, migration, and apoptosis, as well as their correlation with PD-L1 expression.By unraveling the relevant molecular mechanisms, this research will provide a theoretical basis for immune combination therapy and the identification of effective immune therapy response biomarkers in cervical cancer treatment.
2.Materials and Methods
2.1 General Materials
SiHa cells (Cell Bank, Shanghai Academy of Sciences, China); Fetal bovine serum (Gibco); RFect transfection reagents: si - METTL14(sequence of 5 ‘- CCGACAGCATTGGTGCCGTGTTAAA - 3’), si- YTHDF1 (sequence of 5 ‘- CCUACGGACAGCUCAGUAA - 3’),si - NC (sequence: 5 ‘-UUCUCCGAACGUGUCACGUdTdT-3’);CCK-8 assay kit; PCR primer (#YWSJ); and ultra-sensitive chemiluminescence detection kit (all purchased from Nanjing Punoen Biotechnology Co., Ltd); RNA extraction kit; reverse transcription kit (#R333-01); quantitative PCR detection kit (#Q711-02); Annexin V-FITC/PI apoptosis detection kit (all purchased from Nanjing Vazyme Biotech Co.,Ltd); BCA protein concentration determination kit (Beyotime Biotechnology Co., Ltd.); Protein Marker (#01120923) (Thermo Corporation); Rabbit anti-METTL14 Rabbit pAb (#26158-1-AP), YTHDF1 Rabbit pAb (#17479-1-AP), PD-L1/CD274 Rabbit pAb (#28076-1-AP), GAPDH Rabbit pAb (#KK1102) (ProteinTech); HRP Goat Anti-Rabbit IgG(H+L)(#AS014) (ABclonal); Citric acid antigen repair solution (#C1032),rabbit serum (#SL034), DAB chromogenicity kit (#DA1010) (all purchased from Beijing Solarbio Science & Technology Co., Ltd.).
2.2 Methods
2.2.1 Immunohistochemical staining
Paraffin-embedded sections of normal cervical tissue and cervical cancer tissue were deparaffinized in water sequentially.Antigen retrieval, endogenous peroxidase blocking, serum blocking, and overnight incubation with rabbit anti-METTL14 monoclonal antibody (1:300), rabbit anti-YTHDF1 monoclonal antibody (1:300),and PD-L1 monoclonal antibody (1:200) were performed.The next day, HRP-conjugated goat anti-rabbit IgG (1:2 000) was incubated at room temperature for 50 min.DAB chromogenic staining was conducted, followed by counterstaining with hematoxylin,dehydration in neutral gum, and sealing with coverslips.The results were interpreted based on staining observations (cell nuclei stained with hematoxylin appeared blue, and positive expression revealed by DAB staining appeared brownish-yellow).
2.2.2 SiHa cell culture and transfection
SiHa cervical cancer cells were cultured in complete medium containing 10% fetal bovine serum and 1% penicillin-streptomycin at 37 ℃ in a CO2incubator with 5% CO2.One day before transfection, SiHa cells at logarithmic growth phase and with at least two passages were seeded into a 6-well plate.When the cells reached approximately 50% confluence, transfection of small interfering RNA (siRNA) targeting METTL14, siRNA targeting YTHDF1,or negative control siRNA (si-NC) was performed following the transfection reagent’s instructions.The cells were divided into experimental groups (si-METTL14 and si-YTHDF1) and control group (si-NC).After transfection, the cells were incubated in the CO2 incubator for 48 hours for RNA level detection and 72 h for protein level detection.
2.2.3 CCK-8 assay for SiHa cell viability
SiHa cells transfected with si-METTL14, si-YTHDF1 experimental groups (cultured for 0 h, 24 h, 48 h, 72 h, 96 h) and si-NC control group were collected at logarithmic growth phase.The cells were counted and adjusted to a concentration of 30,000 cells/mL.Then,100 μL of cell suspension was seeded into each well of a 96-well plate using a multichannel pipette.Each group had three replicate wells.The plate was incubated at 37 ℃ in a CO2incubator until the cells adhered to the well bottom.Subsequently, 10 μL of CCK-8 solution was added to each well, and the plate was further incubated in the dark for 3 h.The absorbance of each well at 450 nm wavelength was measured using microplate reader.The data was analyzed using GraphPad Prism 8.0.2 software, with time (h) as the x-axis and cell viability (OD450 values) as the y-axis.A growth curve was plotted to visualize the cell growth over time.
2.2.4 qPCR assay for the expression of METTL14, YTHDF1,and PD-L1 mRNA
SiHa cells transfected with si-METTL14, si-YTHDF1 experimental groups, and si-NC control group were collected.Total RNA was extracted according to the instructions of the RNA extraction kit.The concentration and purity of the extracted RNA were measured using microplate reader, followed by reverse transcription.PCR reactions were then performed following the instructions of the quantitative PCR detection kit.GAPDH was used as the internal reference gene with the upstream primer sequence: 5’-GAAGGTGAAGGTCGGAGTC-3’ and downstream primer sequence: 5’-GAAGATGGTGATGGGATTTC-3’.For METTL14, the upstream primer sequence was: 5’-AGTGCCGACAGCATTGGTG-3’ and downstream primer sequence: 5’-GGAGCAGAGGTATCATAGGAAGC-3’.For YTHDF1, the upstream primer sequence was: 5’-AC C T G T C C AG C TAT TAC C C G-3’ a n d d ow n s t r e a m primer sequence: 5’-TGGTGAGGTATGGAATCGGAG-3’.For PD-L1, the upstream primer sequence was: 5’-GCTGCACTAATTGTCTATTGGGA-3’ and downstream primer sequence: 5’-AATTCGCTTGTAGTCGGCACC-3’.
2.2.5 Western blot assay for the expression of METTL14,YTHDF1, and PD-L1 proteins
SiHa cells transfected with si-METTL14, si-YTHDF1 experimental groups, and si-NC control group were collected from the CO2incubator.The cells were lysed with RIPA lysis buffer mixed with PMSF at a ratio of 100:1 to extract total proteins.The protein concentration was quantified using the BCA protein assay kit and normalized accordingly.The extracted proteins were then denatured by heating at 100 ℃.Electrophoresis was performed under a constant voltage of 150V for approximately 90 min until the bromophenol blue dye ran to the bottom of the gel.The proteins were then transferred onto a PVDF membrane at a constant current of 250 mA for 1 h.The membrane was blocked with 5% skim milk for 1 h.Next, rabbit anti-METTL14 monoclonal antibody (1:1 500), rabbit anti-YTHDF1 monoclonal antibody (1:4 000), rabbit anti-PD-L1 monoclonal antibody (1:600), and rabbit anti-GAPDH monoclonal antibody (1:1 000) were added to the PVDF membrane and incubated overnight at 4 ℃.The following day, the PVDF membrane was washed to remove the primary antibodies, and HRPconjugated goat anti-rabbit IgG(H+L) (1:5 000) was added to the membrane, followed by incubation at room temperature for 1 h.After washing the membrane, a highly sensitive ECL chemiluminescence solution was prepared, and the chemiluminescent images were captured using a chemiluminescence imaging system.
2.2.6 Transwell assay to assess SiHa cell migration ability
After transfection, SiHa cells from the experimental groups (si-METTL14 and si-YTHDF1) and the control group (si-NC) were collected.The cells were then digested with trypsin, counted, and adjusted to a cell density of 300,000 cells/mL.Next, 200μL of the cell suspension was added to the upper chamber of the Transwell insert, and 600μl of DMEM culture medium containing 20% FBS was added to the lower chamber.The Transwell inserts were placed in a 37 ℃, 5% CO2incubator and incubated for 48 h to allow cell migration.After the incubation period, any non-migrating cells on the upper side of the Transwell insert were gently wiped off using a dry medical cotton swab.The migrated cells on the lower side of the membrane were fixed with 4% paraformaldehyde solution at room temperature for 20 min.Subsequently, the cells were stained with crystal violet solution at room temperature in the dark for 15 minutes.After washing with PBS, the Transwell inserts were allowed to air dry naturally.The migrated cells were visualized under a microscope, and images were captured.ImageJ software was then used to quantify the number of migrated cells.Data analysis and graphical representation were performed using GraphPad Prism 8.0.2 software.
2.2.7 Flow cytometry to assess SiHa cell apoptosis
SiHa cells from the experimental groups (si-METTL14 and si-YTHDF1) and the control group (si-NC) were collected and digested with trypsin.The cells were then washed twice with prechilled PBS, centrifuged, and resuspended in an appropriate volume of 1× Binding Buffer to achieve a cell density of 1×106cells/ml.Experimental groups were set up, including blank tubes, FITC single-stained tubes, PI single-stained tubes, and FITC-PI doublestained tubes.Control groups were also set up, including blank tubes and FITC-PI double-stained tubes.Each tube was loaded with 100 μL of cell suspension.For single-stained tubes, 5 μL of FITC and 5 μL of PI were added separately.For the double-stained tubes, 5 μL of FITC and 5 μL of PI were added simultaneously.The tubes were incubated at room temperature in the dark for 15 min.After incubation, 400 μL of 1x Binding Buffer was added to each tube, and the contents were thoroughly mixed.The tubes were then analyzed using a flow cytometer to assess the apoptosis status of the cells.The results were subsequently analyzed using FlowJo 10 software.
3.Results
3.1 High expression of METTL14, YTHDF1, and PD-L1 in cervical cancer tissues
Immunohistochemical staining was performed on cervical cancer tissues and normal cervical tissues.The results revealed that compared to normal cervical tissues, METTL14, YTHDF1, and PDL1 were significantly upregulated in all 8 cervical cancer samples(refer to Figure 1).
3.2 Knockdown of METTL14 and YTHDF1 inhibits SiHa cell viability
To investigate the role of METTL14 and YTHDF1 in cervical cancer, the expression of METTL14 and YTHDF1 was knocked down in SiHa cells using specific siRNAs, along with negative control and fluorescence control transfections.The transfection efficiency was observed using fluorescence electron microscopy(refer to Figure 2A), and the knockdown efficiency was further confirmed by qPCR (P<0.05, refer to Figure 2B).After knocking down METTL14 and YTHDF1, the effect on SiHa cell growth was assessed using the CCK-8 assay.The results demonstrated that the knockdown of METTL14 and YTHDF1 significantly decreased SiHa cell viability (P<0.05, refer to Figure 2C).

Fig 1 Immunohistochemical staining of METTL14, YTHDF1, and PD-L1 expression levels in cervical tissues

Fig 2 The impact of METTL14 and YTHDF1 knockdown on SiHa cell viability A: Fluorescence microscopy observation of knockdown efficiency.B: Realtime quantitative PCR validation of knockdown efficiency.C: CCK-8 assay to detect SiHa cell viability
3.3 Knockdown of METTL14 and YTHDF1 inhibits SiHa cell migration
To further investigate the impact of METTL14 and YTHDF1 on cervical cancer cell migration, SiHa cells were transfected with specific siRNAs targeting METTL14 and YTHDF1, and their migration ability was assessed using the Transwell cell migration assay.The results showed a significant reduction in the number of migrated cells in the si-METTL14 and si-YTHDF1 groups compared to the si-NC group (P<0.05, refer to Figure 3).This indicates that knockdown of METTL14 and YTHDF1 significantly inhibits the migration ability of SiHa cervical cancer cells.
3.4 Knockdown of METTL14 and YTHDF1 promotes apoptosis in SiHa cells
To investigate whether METTL14 and YTHDF1 affect apoptosis in SiHa cells, flow cytometry was used to assess the apoptosis status after knocking down METTL14 and YTHDF1.The results showed a significant increase in the number of apoptotic SiHa cells in the si-METTL14 and si-YTHDF1 groups compared to the si-NC group (P<0.05, refer to Figure 4).This indicates that knockdown of METTL14 and YTHDF1 promotes apoptosis in SiHa cells.
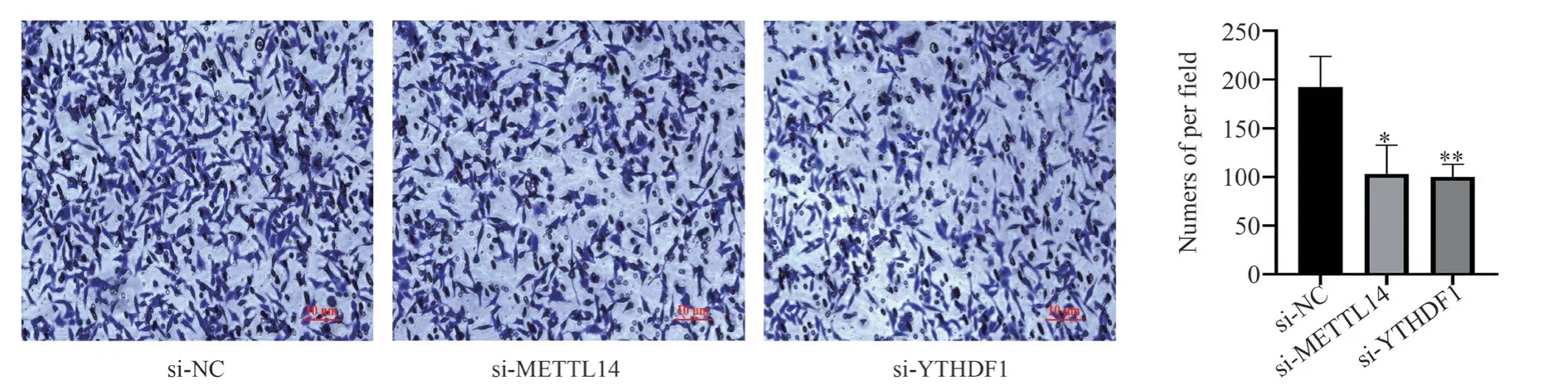
Fig 3 The effect of METTL14 and YTHDF1 knockdown on SiHa cell migration
3.5 Knockdown of METTL14 and YTHDF1 inhibits PD-L1 expression
The impact of METTL14 and YTHDF1 knockdown on PD-L1 mRNA expression was evaluated using qPCR.The results showed a significant downregulation of PD-L1 mRNA expression after knocking down METTL14 and YTHDF1 (P<0.05, refer to Figure 5A, 5B).Furthermore, Western blot analysis was performed to determine whether the knockdown of METTL14 and YTHDF1 affects PD-L1 protein expression.The results demonstrated a significant reduction in PD-L1 protein levels in response to the knockdown of METTL14 and YTHDF1 (P< 0.05, refer to Figure 5C, 5D).

Fig 4 The effect of METTL14 and YTHDF1 knockdown on SiHa cell apoptosis Note: The lower-left quadrant represents normal cells, the upper-left quadrant represents mechanically injured normal cells, the lower-right quadrant represents early apoptotic cells, and the upper-right quadrant represents late apoptotic cells
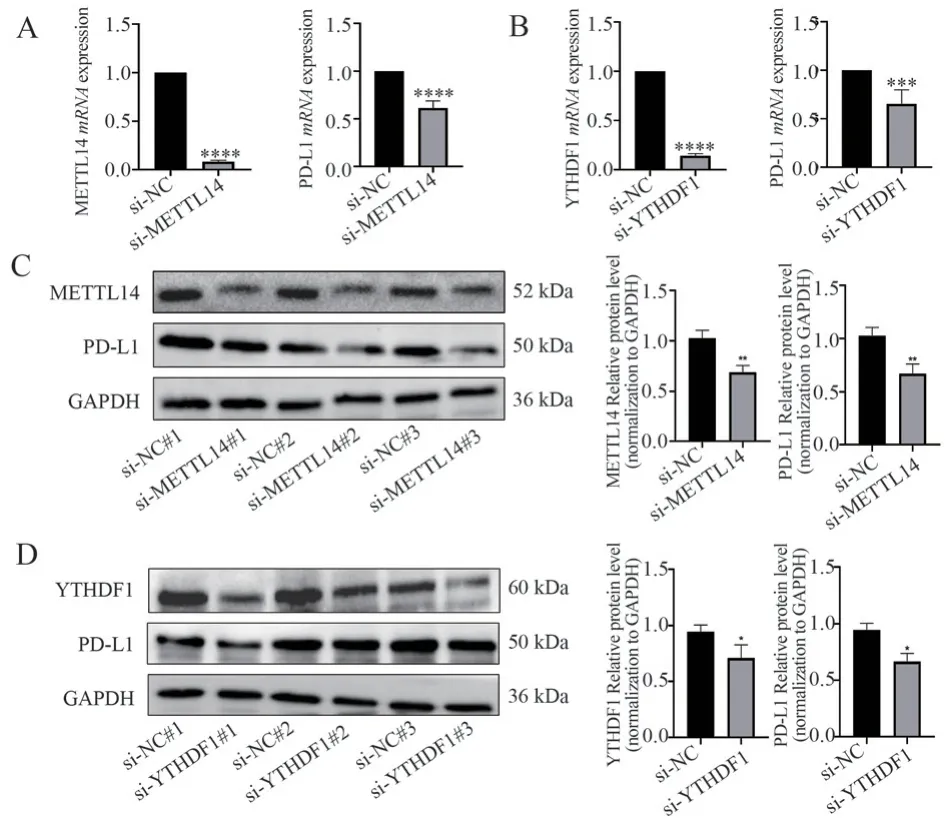
Fig 5 The effect of METTL14 and YTHDF1 knockdown on PD-L1 expression A: The mRNA expression level of PD-L1 after METTL14 knockdown.B: The mRNA expression level of PD-L1 after YTHDF1 knockdown.C: The protein expression level of PD-L1 after METTL14 knockdown.D: The protein expression level of PD-L1 after YTHDF1 knockdown
4.Discussion
Traditional treatments for cervical cancer mainly involve surgery,radiotherapy, and chemotherapy.In recent years, some new targeted therapies have also been used in the treatment of cervical cancer,such as targeting vascular endothelial growth factor and epidermal growth factor receptors.These drugs can more precisely target cervical cancer cells, reducing harm to normal cells[8].Additionally,novel treatment approaches developed using new technologies like artificial intelligence, gene editing, and nucleic acid delivery are also under extensive research[9].Furthermore, with the approval and market availability of the first PD-1 inhibitor, pembrolizumab,as well as domestically produced cost-effective PD-1 inhibitors,immunotherapy has become a prominent focus in cervical cancer treatment[10].Researchers have also analyzed tissue and blood samples from cervical cancer patients and identified the significant roles of epigenetic modifications, gene mutations, and other factors in the development of cervical cancer.They have also identified a series of potential biomarkers, offering new targets and approaches for cervical cancer treatment[11].In this study, the expression of m6A modification-related regulatory genes METTL14 and YTHDF1, as well as the immune checkpoint PD-L1, in cervical cancer tissues and normal cervical tissues, was observed using immunohistochemistry.The significant overexpression of METTL14, YTHDF1, and PD-L1 in cervical cancer tissues suggests a potential correlation between m6A-related genes and immune checkpoints, which may have a significant impact on cervical cancer progression and prognosis.
m6A methylation modification can regulate the progression of diseases and has different regulatory effects in different tumors,attracting extensive attention in the field of tumor treatment[12].METTL14 is one of the main methyltransferases that catalyze m6A modification.It plays a crucial role in catalyzing m6A methylation on RNA, including aspects such as stability, splicing, transportation,and translation[13].Studies have found that METTL14 promotes the m6A-dependent degradation of Siah2, an E3 ubiquitin ligase, in cholangiocarcinoma.Siah2 expression suppresses PD-L1 expression,and tumors with low Siah2 expression are more sensitive to PD-1 inhibitor therapy, thus confirming the potential role of the METTL14-Siah2-PD-L1 regulatory axis in cancer immunotherapy[14].Moreover, research has shown that combined treatment with PD-1 inhibitors and METTL14 silencing effectively inhibits tumor progression and enhances the efficacy of immunotherapy.The main mechanism is that inhibiting METTL14 can alter the recruitment of CD8+TIL in the tumor microenvironment, making cancer patients more sensitive to immunotherapy[15].YTH domain family protein 1 (YTH N6-Methyladenosine RNA Binding Protein F1, YTHDF1)is an m6A modification-related RNA binding protein that regulates mRNA translation by binding to m6A on mRNA[16].Studies have confirmed that YTHDF1-deficient mice exhibit stronger presentation of tumor-specific antigens and activation of CD8+T cells compared to wild-type mice, resulting in a more durable anti-tumor response.Additionally, downregulation of YTHDF1 combined with PD-1 inhibitors can regulate the expression of lysosomal proteases in dendritic cells through an m6A-dependent mechanism, thus enhancing the therapeutic effect of immune checkpoint inhibitors[17].AlkB homolog 5 (ALKBH5), as an important m6A demethylase,was found to directly target m6A modification on PD-L1 mRNA in m6A methylome profiling.Intrinsic ALKBH5 in tumors can inhibit the expansion of T cells by maintaining the expression of PD-L1 in tumor cells, thereby promoting tumor progression[18].These results confirm the feasibility of targeting m6A in immunomodulatory therapies and represent a new approach to overcoming tumor immune resistance.Targeted tumor immunotherapy has achieved promising results in cancer treatment, but in the treatment of cervical cancer, it is still in the development stage.Therefore, it is necessary to explore potential targets and effective combination methods for the benefit of cervical cancer immunotherapy in-depth.
This study first observed significant upregulation of m6A modification-related genes METTL14, YTHDF1, and immune checkpoint PD-L1 in cervical cancer tissues, with their expression levels positively correlated.Subsequently, the expression of METTL14 and YTHDF1 genes was knocked down, and their effects on cervical cancer cell functions were validated through CCK-8,Transwell, and flow cytometry experiments.The results showed that knockdown of METTL14 and YTHDF1 significantly inhibited the proliferation and migration of SiHa cells while promoting apoptosis.Additionally, the correlation between METTL14, YTHDF1, and PD-L1 was verified by qPCR and Western blot, demonstrating that PD-L1 expression decreased with the downregulation of METTL14 and YTHDF1.Therefore, this study concludes that m6Arelated regulatory factors METTL14 and YTHDF1 significantly influence the functions of cervical cancer cells and promote the occurrence, development, and immune resistance of cervical cancer by upregulating PD-L1.The specific mechanisms underlying these effects require further in-depth investigation.This study also speculates that METTL14 and YTHDF1 could be potential effective targets for targeted tumor immunotherapy and may serve as biomarkers for identifying potential beneficiaries of cervical cancer immunotherapy.In the future, small molecule inhibitors targeting METTL14 or YTHDF1 in combination with PD-1 inhibitors could be used for cervical cancer treatment, or the expression of METTL14 and YTHDF1, along with PD-L1, could be detected to screen for individuals who may benefit from immunotherapy.
Authors’ Contributions
Minmin Yu: Responsible for experimental design, experimental guidance and paper revision; Jingrui Yang: Responsible for experimental implementation, data processing and paper writing;Nana Xia: Responsible for part of the experiment implementation and data processing.
 Journal of Hainan Medical College2023年19期
Journal of Hainan Medical College2023年19期
- Journal of Hainan Medical College的其它文章
- Study on the in vitro anti ovarian cancer effect and mechanism of quinazoline derivative (N111)
- Expression and correlation of pyroptosis-related markers and PI3K/AKT pathway in endometriosis
- Meta-analysis of the efficacy of volar plate internal fixation versus closed reduction and external fixation in the treatment of adult distal radius fractures
- A review of the epidemic and clinical study on scrub typhus in China(2010-2020)
- MiR-15a-5p in neutrophil exosomes promotes macrophage apoptosis through targeted inhibition of BCL2L2
- Clinical efficacy of bushen huatan huoxue recipe in combination with acupuncture in treating patients suffering from polycystic ovary syndrome with insulin resistance
