Evaluation of Six Recombinant Proteins for Serological Diagnosis of Lyme Borreliosis in China*
LIU Wei, LIU Hui Xin, ZHANG Lin, HOU Xue Xia, WAN Kang Lin, and HAO QinState Key Laboratory for Infectious Diseases Prevention and Control, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China;Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Hangzhou 310003, Zhejiang,China
Original Article
Evaluation of Six Recombinant Proteins for Serological Diagnosis of Lyme Borreliosis in China*
LIU Wei, LIU Hui Xin, ZHANG Lin, HOU Xue Xia, WAN Kang Lin#, and HAO Qin#
State Key Laboratory for Infectious Diseases Prevention and Control, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China;Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Hangzhou 310003, Zhejiang,China
Abstract
Objective In this study, we evaluated the diagnostic efficiency of six recombinant proteins for the serodiagnosis of Lyme borreliosis (LB) and screened out the appropriate antigens to support the production of a Chinese clinical ELISA (enzyme-linked immunosorbent assay) kit for LB.
Methods Six recombinant antigens, Fla B.g, OspC B.a, OspC B.g, P39 B.g, P83 B.g, and VlsE B.a, were used for ELISA to detect serum antibodies in LB, syphilis, and healthy controls. The ELISA results were used to generate receiver operating characteristic (ROC) curves, and the sensitivity and specificity of each protein was evaluated. All recombinant proteins were evaluated and screened by using logistic regression models.
Results Two IgG (VlsE and OspC B.g) and two IgM (OspC B.g and OspC B.a) antigens were left by the logistic regression model screened. VlsE had the highest specificity for syphilis samples in the IgG test(87.7%, P<0.05). OspC B.g had the highest diagnostic value in the IgM test (AUC=0.871). Interactive effects between OspC B.a and Fla B.g could reduce the specificity of the ELISA.
Conclusion Three recombinant antigens, OspC B.g, OspC B.a, and VlsE B.a, were useful for ELISAs of LB. Additionally, the interaction between OspC B.a and Fla B.g should be examined in future research.
Lyme borreliosis; Recombinant proteins; Serological diagnosis; Logistic regression; ROC
www.besjournal.com (full text) CN: 11-2816/Q Copyright ©2016 by China CDC
INTRODUCTION
Lyme borreliosis (LB), a tick-borne zoonotic disease, is caused by Borrelia burgdorferi infection[1]. Many studies have demonstrated that B. burgdorferi has at least 15 genospecies, four of which are associated with human disease[2-6]. The major pathogen of LB is B. burgdorferi sensu stricto in the USA, while B. afzelii and B. garinii are the most frequently reported pathogens in Europe and China[2-6]. In China, a human LB case was first reported in 1985 in a forest region in Hailin County, Heilongjiang province. LB cases have been observed in 29 Chinese provinces/municipalities in 30 years. The major endemic areas are in the north forests of China. Based on a random sampling survey in 20 provinces,the serological positivity of LB was 5.06% (1.06%-12.8%) and the morbidity was 2.84% (1.16%-4.51%)[7].
LB is a multisystem illness. The early disease usually begins with erythema migrans (EM)skin lesions, the most common clinical manifestation. With disease progression, the spirochete might disseminate to other organs, leading to arthritis, neuroborreliosis, carditis, lymphocytoma,etc.[8-9]
Culture is still the gold standard in the laboratory diagnosis of LB, but its low detection rate and long assay time are not suitable for clinical practice[10-13]. Therefore, the diagnosis of LB is often based on the typical clinical manifestation of EM. In the absence of EM, laboratory serodiagnosis should be used. In the United States and parts of Europe, a two-step approach is recommended for LB serodiagnosis. The first step involves ELISA (enzyme-linked immunosorbent assay) or IFA (immunofluorescence antibody assay), which are used for initial screening, and the second step uses western blotting for reactive or equivocal samples obtained in the first step[8,14-15]. In China, a standard detection kit is still lacking. The whole cell proteins of B. burgdorferi are often used in the first step, but the whole cell bacterial protein cross-reacts with proteins from other bacteria, such as Microspironema pallidum and HGE (Human Granulocytic Ehrlichia)[16-18]. Many recombinant proteins have been examined to improve the specificity and sensitivity of Lyme serological tests,including OspC, Fla, P39, VlsE, BBK32, P37, P22,DbpA, P58, P18, OspA, etc.[16-17,19-21].
In this study, the diagnostic efficiency of six recombinant antigens of Chinese B. burgdorferi isolates were evaluated to identify the most appropriate antigen for LB serodiagnosis.
MATERIALS AND METHODS
Serum Samples
Three panels of serum samples were used in this study. The first panel of serum samples was obtained from LB patients at MuDanjiang Linye Hospital (Heilongjiang Province, China, 2012-2013). Clinical diagnosis of LB was based on the following conditions: 1, history of a tick bite; 2, EM, expanding red ≥5 cm in diameter, with or without central clearing; if <5 cm in diameter, a delay in appearance after the tick bite of at least 2 days, and an expanding rash at the site of the tick bite; 3, IFA or ELISA (positive, +); 4, western blot (positive, +); 5,other symptoms of LB, such as arthritis, carditis, and neuroborreliosis[8,22]. All participants who met conditions 1, 2, and 3 or conditions 1, 3, 4, and 5 were diagnosed as LB. Sample characteristics are summarized in Table 1. A total of 139 serum samples from LB patients, including 67 IgM and 72 IgG seropositive samples were used for ELISA. The second panel included 90 serum samples from healthy donors as controls. The third panel had 89 specimens obtained from syphilis patients at Urumqi First People's Hospital (Xinjiang Uygur Autonomous Region, China). These specimens were diagnosed as syphilis based on clinical manifestations and TPHA (Treponema pallidum hemagglutination) tests. All serum samples were stored at -80 °C before tests and each sample was divided into 3 Eppendorf tubes to minimize the loss of antibodies during freezing and thawing.
This study was approved by the Ethical Review Committee of the National Institute for Communicable Disease Control and Prevention (ICDC), Chinese Center for Disease Control and Prevention (China CDC). Participants also provided written informed consent to participate in the study.
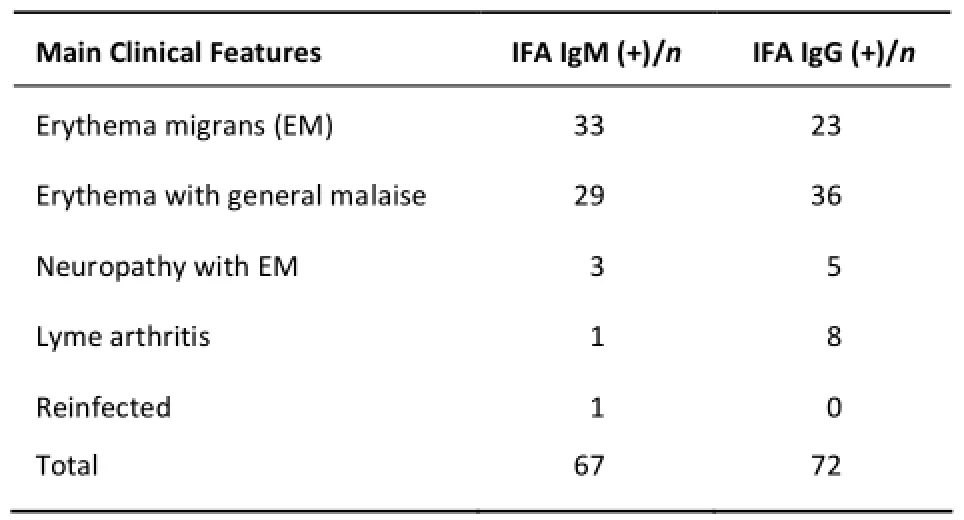
Table 1. Characterization of Lyme Borreliosis Serum Samples Used in the Study
Antigens
Six proteins were examined in this study, four of which (P39 B.g, P83 B.g, OspC B.g, and Fla B.g) were cloned and expressed as described previously[23-26]. Primers for these antigens were described in previous studies[23-26]with slight modifications. In addition, a conserved region of VlsE from B. afzelii strain GDsh1 was expressed. This conserved region was identified in all VlsE sequences of B. afzelii strains in PubMed and was expressed for the first time in our study. Another protein, OspC B.a, and two B. burgdorferi strains used in this study were provided by the Department of Lyme borreliosis,National Institute for Communicable Disease Controland Prevention, Chinese Center for Disease Control and Prevention (China CDC). B. burgdorferi strain PD91 was isolated from LB patients and GDsh1 was isolated from Rattus norvegicus. These strains were genotyped by a multilocus sequence analysis (MLSA). A whole cell ELISA kit was constructed using the PD91 strain. All primers and strains for candidate antigens are described in supplementary material Table S1.
ELISAs with Recombinant Antigens
Recombinant antigens and whole cells of B. burgdorferi were used to detect serum IgG and IgM antibodies by class-specific ELISAs. The optimal concentrations of all proteins were determined by checkerboard titrations[17]. The most appropriate serum dilution was 1:50, except for OspC at 1:100. The γ-chain-specific affinity-purified horseradish peroxidase-labeled goat anti-human IgG antibodies (Sigma, St. Louis, MO, USA) were diluted at 1:8000,while the µ-chain-specific affinity-purified horseradish peroxidase-labeled goat anti-human IgM antibodies (Sigma) were diluted at 1:10,000. Details of the blocking, incubation, and washing procedures,the substrate, and the measurement of absorbance values have been reported previously[17].
For each antigen, receiver operating characteristic (ROC) curves were generated using the results for LB sera and healthy controls, and the area under the ROC curve (AUC) was estimated. The cut-off value for each antigen was determined by the maximum Youden index of the ROC curve evaluated using MedCalc software.
Statistical Analysis
Logistic regression and Chi square tests were used to evaluate the efficiency of the 6 antigens with respect to the serum detection of IgG and IgM antibodies, and all statistical analyses were performed in MedCalc and SAS. Z-statistics were obtained from the pairwise comparisons of ROC curves in MedCalc (Z=Difference between areas/Standard Error).
RESULTS
Sensitivity and Specificity of the Six Recombinant Antigens
Recombinant proteins were used to detect serum IgG and IgM from three panels. The ROC curves for the antigens are displayed in Figure 1.
IgM Antibody Cutoff values for antigens in the detection of IgM based on the ROC curves were as follows: Fla B.g 0.083, OspC B.a 0.063, OspC B.g 0.087, P39 B.g 0.052, P83 B.g 0.053, VlsE B.a 0.065, and whole cell proteins 0.07. The sensitivity ranged from 61.2% to 95.5% and specificity ranged from 60% to 88% (Table 2). In a comparison of diagnostic values, OspC B.g had a higher diagnostic value than P39 B.g, P83 B.g, and VlsE B.a (P<0.05). In addition, P39 had the lowest diagnostic value (P<0.05) among all proteins (Table 3). For the 89 syphilis serum samples, VlsE B.a, Fla B.g, and OspC B.g had high specificities, and no significant difference was detected between the three proteins (P>0.05).
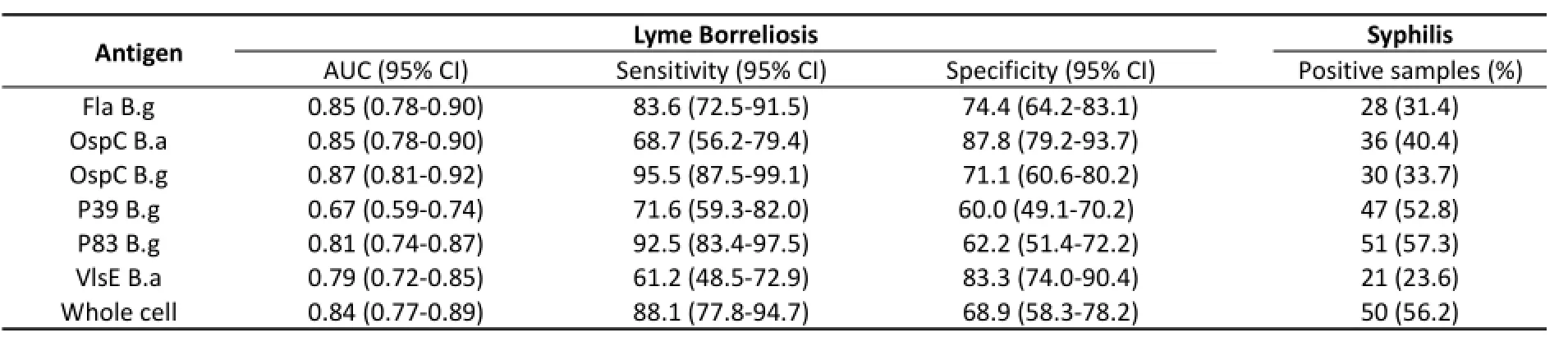
Table 2.Summary of IgM Antibody Detection in The Serum of Borrelia burgdorferi for Various Antigens in Patients with Lyme Borreliosis and Syphilis
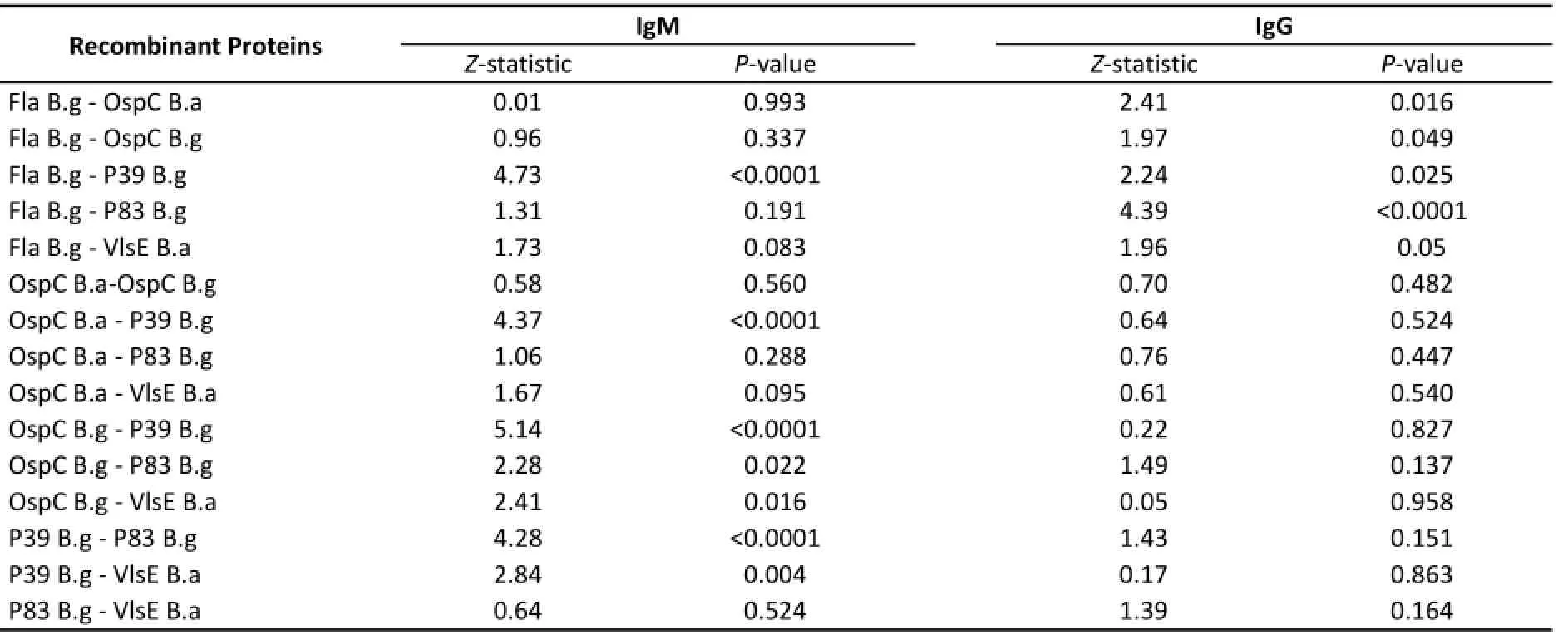
Table 3.Pairwise Comparison of ELISA Results for 6 Recombinant Proteins in LB Serum Samples and Healthy Controls
IgG AntibodyCutoff values for the detection of IgG using different antigens were as follows:Fla B.g 0.164,OspC B.a 0.37,OspC B.g 0.16,P39 B.g 0.17,P83 B.g 0.2,VlsE B.a 0.16,and whole cell proteins 0.16.The sensitivity ranged from 56.9%to 88.9%, and specificity ranged from 58.9%to 78.9%(Table 4). Based on Z‐statistics,Fla B.g had a higher diagnostic value than the other proteins(P<0.05)(Table 3).For syphilis serum samples,Fla B.g had the highest positive detection rate(P<0.05).VlsE B.a and OspC B.a had high specificity values for syphilis serum,and no significant difference was detected between the two proteins(P>0.05).
Evaluation of the Effectiveness of Recombinant Antigens
Two logistic regression models were established for IgM and IgG.The effectiveness of each protein was evaluated for the detection of two antibodies. OspC B.a and OspC B.g had high diagnostic values for IgM,while OspC B.g and VlsE B.a were effective for the diagnosis of IgG(Table 5).The specificity of VlsE B.a in the test of syphilis IgG was much higher than that of OspC B.g(Table 4).Therefore,VlsE B.a was the most effective antigen for the diagnosis of IgG.
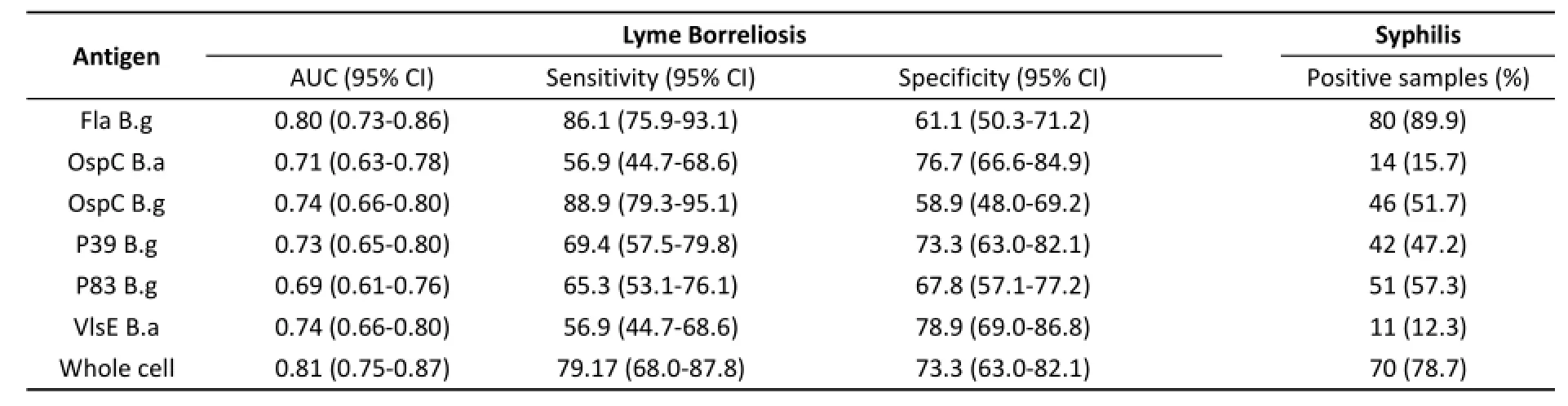
Table 4.Summary of IgG Antibody Detection of Borrelia burgdorferi using Various Antigens in Patients with Lyme Borreliosis and Syphilis
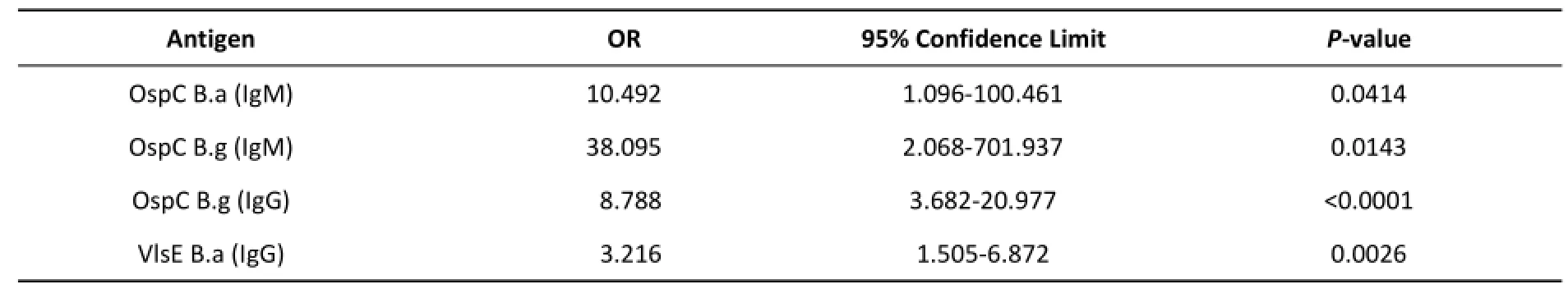
Table 5.Results of Reduced Logistic Regression Models
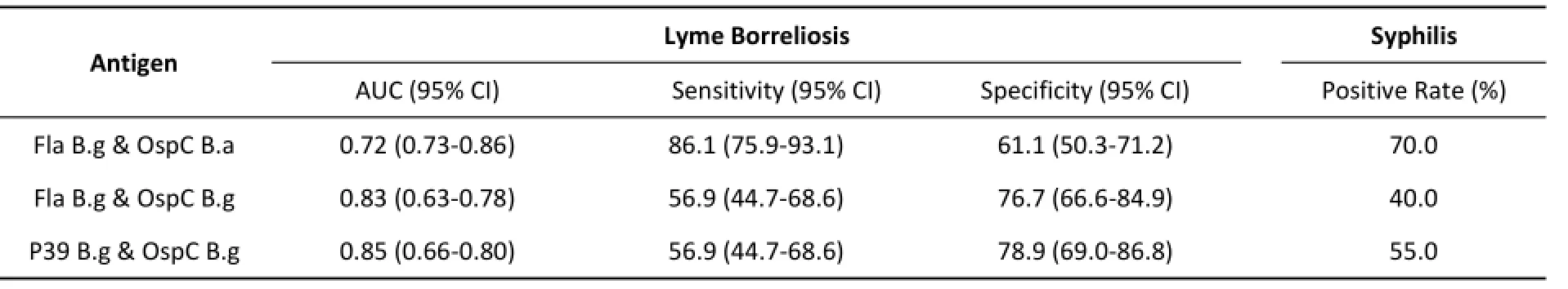
Table 6.IgM ELISA Results Using Mixed Antigens for Various Serum Panels

Table 7.Pairwise Comparison of Screened Proteins and Whole Cell Proteins in Syphilis Serum Samples
Interactions between antigens were analyzed by logistic regression models.Interestingly,there was no significant interaction in the IgG test.However,for IgM,three groups(Fla B.g and OspC B.a,Fla B.g and OspC B.g,and OspC B.g and P39 B.g)were found had interactions in model.The pairs of proteins were mixed and used for ELISA with the three serum panels.The results are shown in Table 6.There was no difference between the mixed proteins and OspC B.g(P>0.05),and the specificity of OspC B.g was higher than that of the mixed proteins in the syphilis test(P<0.05).The diagnostic value of Fla B.g with OspC B.a was lower than those of the single antigens.
Comparison between Diagnoses Using the Screened Recombinant Antigens and The Whole Cell Proteins of Borrelia burgdorferi
The whole cell proteins of B.g strain PD91 was used to detect IgG and IgM in the three serum panels.In terms of OspC B.g IgM,OspC B.g IgG,OspC B.a IgM,and VlsE B.a IgG,there were no differences in the ELISA results between screened antigens and whole cell proteins for LB serum samples and healthy controls(in Figures S1 and S2).However,using syphilis serum samples,the specificity values for the single screened proteins were higher than that of the whole cell(Chi‐square tests,P<0.05,data shown in Table 7).In particular,OspC and VlsE were better than the whole cell proteins for the diagnosis of LB.
DISCUSSION
B. garinii and B. afzelii are the main pathogenic species of Borrelia burgdorferi in China. These pathogens have mainly been found in Liaoning Province, Jilin Province, Heilongjiang province, and the Inner Mongolia Autonomous Region[4]. In this study, we obtained patient serum samples from Heilongjiang province and used strains isolated from the endemic area of B. burgdorferi as templates to clone and express 6 antigens for serological evaluations. VlsE and the VlsE IR6 peptide are frequently applied in serological tests to detect LB antibodies. The high sensitivity and specificity in serum diagnosis can distinguish between LB and syphilis[6,16,27]. However, VlsE is a variant protein of the Vmp (variable major protein) system, and its high variation among strains makes in vivo expression difficult[28]. In this study, we used a Chinese strain as the template and cloned and expressed a conserved segment of VlsE for ELISA. Fla is an effective antigen for the serodiagnosis of early LB using ELISA based on previous analyses[29]. OspC (23 kD) is a molecule with antigenic diversity; it is expressed in the early stage of infection and is associated with the escape of host immunity[30-31]. As a kind of immune-dominant antigen, it is often used to detect IgM for early LB[30-33]. P83/100 and P39 are also commonly used in studies of late LB[34-35]. The P83/100 protein is an immunodominant protein associated with the immune escape mechanism of B. burgdorferi[36-37]. This protein exists in 20% of patients with early LB and almost all advanced and late-stage patients[38]. The E4 region of P83 is specifically used for the serodiagnosis of LB[39]and was used in our study. Borrelia membrane protein A (BmpA), also called P39, is a specific diagnostic antigen for LB[40]. These antigens are detected at different stages of B. burgdorferi infection, and have been studied for use in tests. However, a comprehensive evaluation of recombinant proteins for LB serodiagnosis in China is lacking. We used these antigens to detect antibodies in serum samples at different stages of LB in Chinese patients.
Using the three serum panels for IgG detection,Fla B.g had the best diagnostic value for LB patients and healthy controls, but it also had the highest positive rate using syphilis sera, consistent with the results of a previous report[18]. Fla had poor specificity in this study, consistent with a previous study indicating that it has high cross-reactivity in serologic tests with similar antigens expressed by other bacteria. The specificity of VlsE in the syphilis test was much higher than that of other proteins. According to the reduced logistic regression model,three proteins, OspC B.g, OspC B.a, and VlsE B.a,were left. Combined with the results obtained using syphilis sera, VlsE B.a and OspC B.g for the IgG test and OspC B.g and OspC B.a for the IgM test were optimal for LB diagnosis. In this study, there was no difference between single and mixed proteins. Interactions between the proteins are not clear. The sensitivity and specificity of the whole cell proteins were similar to those of the single proteins for ELISA using LB sera and healthy controls, but the specificity of single proteins was much higher in syphilis serum samples.
In conclusion, we screened three recombinant antigens for LB serum diagnosis, OspC B.g, OspC B.a,and VlsE B.a. An unexpected discovery was that the interaction between Fla B.g and OspC B.a might decrease the diagnostic value in the LB test. This research provides a strong basis for the production of a recombinant antigen ELISA kit for LB serum antibody detection in China.
This study had some limitations. Most of the LB serum samples were obtained from EM patients, but the proportion of advanced and late-stage LB serum samples was small. Evaluations of the efficiency of antigens at different disease stages will improve accuracy. In addition, additional antigens or specific peptides should be considered for the detection of LB serum antibodies in the future.
Accepted: April 25, 2016
REFERENCES
1. Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. New Engl J Med, 1983; 308, 733-40.
2. Bacon RM, Kugeler KJ, Griffith KS, et al. Lyme disease-United States, 2003-2005. MMWR Morb Mort Wkly Rep, 2007; 56,573-6.
3. Stanek G, Reiter M. The expanding Lyme Borrelia complex-clinical significance of genomic species. Clin Microbiol Infect, 2011; 17, 487-93.
4. Hao Q, Hou X, Geng Z, et al. Distribution of Borrelia burgdorferi sensu lato in China. J Clin Microbiol, 2011; 49, 647-50.
5. Chu CY, Jiang BG, He J, et al. Genetic diversity of Borrelia burgdorferi sensu lato isolates from Northeastern China. Vector-Borne Zoonot, 2011; 11, 877-82.
6. Marques AR. Laboratory Diagnosis of Lyme Disease: Advances and Challenges. Infect Dis Clin North Am, 2015; 29, 295-307.
7. Wu XB, Na RH, Wei SS, et al. Distribution of tick-borne diseases in China. Parasites & Vectors, 2013; 6, 1-8.
8. Stanek G, Fingerle V, Hunfeld KP, et al. Lyme borreliosis:clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect, 2011; 17, 69-79.
9. Arvikar SL, Steere AC. Diagnosis and Treatment of Lyme Arthritis. Infect Dis Clin North Am, 2015; 29, 269-80.
10.Aguero-Rosenfeld ME. Lyme disease: laboratory issues. Infect Dis Clin North Am, 2008; 22, 301-13.
11.Strle F, Ružić-Sabljić E, Cimperman J, et al. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis, 2006; 43,704-10.
12.Wilske B, Fingerle V, Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol, 2007; 49, 13-21.
13.Brouqui P, Bacellar F, Baranton G, et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect, 2004; 10, 1108-32.
14.URL 2007. Societe de Pathologie Infectieuse de Langue Francaise(SPILF). Lyme borreliosis: diagnosis, treatment and prevention. 16thconsensus conference on anti-infective therapy. http: // www.infectiologie.com/site / medias/english /Lyme_shortext-2006.pdf
15.Hunfeld KP, Kraiczy P. When is the best time to order a western blot and how should it be interpreted.Curr Probl Dermatol, 2009; 37, 169-79.
16.Gomes-Solecki MJC, Meirelles L, Glass J, et al. Epitope length,genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin Vaccine Immunol, 2007;14, 875-9.
17.Magarelli LA, Lawrenz M, Norriss J, et al. Comparative reactivity of human sera to recombinant VlsE and other Borrelia burgdorferi antigens in class-specific enzyme-linked immunosorbent assays for Lyme borreliosis. J Med Microbiol,2002; 51, 649-55.
18.Magnarelli LA, Anderson JF, Johnson RC. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis, 1987; 156, 183-8.
19.Panelius J, Lahdenne P, Saxén H, et al. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VlsE IR6 peptide. J Neurol, 2003;250, 1318-27.
20.Göttner G, Schulte-Spechtel U, Hillermann R, et al. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J Clin Microbiol, 2005; 43, 3602-9.
21.Dessau RB, Møller JK, Kolmos B, et al. Multiplex assay (Mikrogen recomBead) for detection of serum IgG and IgM antibodies to 13 recombinant antigens of Borrelia burgdorferi sensu lato in patients with neuroborreliosis: the more the better? J Med Microbiol, 2015; 64, 224-31.
22.Stanek G, Strle F. Lyme borreliosis. Lancet, 2012; 379, 461-73.
23.Chen J, Wan KL. Recombinant OspC identification and antigenicity detection from Borrelia burgdorferi PD91 in China. Chinese Journal of Epidemiology, 2003; 24, 917-9. (In Chinese)
24.YuPin T, FangBo F, ShengLi B. Molecular cloning and expression of Borrelia burgdorferi P39 gene in E. coli. Chinese Journal of Zoonoses, 2000; 16, 9-11. (In Chinese)
25.Bao L, Xie Y. PCR amplification and cloning of the specific region of the gene encoding 83kd antigen from Borrelia burgdorferi. Journal of West China University of Medical Sciences, 1999; 30, 5-7. (In Chinese)
26.Lu B, Wan K. Cloning and expression of the central fragment of flagellin gene from the Chinese strain PD91 of Borrelia garinii. Chinese Journal of Micobiology and Immunology, 2004; 24,611-4. (In Chinese)
27.Pomelova VG, Korenberg EI, Kuznetsova TI, et al. C6 Peptide-Based Multiplex Phosphorescence Analysis (PHOSPHAN) for Serologic Confirmation of Lyme Borreliosis. PLoS One, 2015; 10, e0130048.
28.Göttner G, Schulte-SpechtelU, Wilske B. Heterogeneity of the immunodominant surface protein VlsE among the three genospecies of Borrelia burgdorferi pathogenic for humans. Int J Med Microbiol, 2004; 293, 172-3.
29.Panelius J, Lahdenne P, Saxen H, et al. Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J Clin Microbiol,2001; 39, 4013-9.
30.Xu Q, Seemanapalli SV, McShan K, et al. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity[J]. Infect Immun, 2006; 74, 5177-84.
31.Liang FT, Jacobs MB, Bowers LC, et al. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med, 2002; 195, 415-22.
32.Embers ME, Alvarez X, Ooms T, et al. The failure of immune response evasion by linear plasmid 28-1-deficient Borrelia burgdorferi is attributable to persistent expression of an outer surface protein. Infect Immun, 2008; 76, 3984-91.
33.Arnaboldi PM, Seedarnee R, Sambir M, et al. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early Lyme disease. Clin Vaccine Immunol, 2013; 20, 474-81.
34.Hauser U, Lehnert G, Wilske B. Diagnostic Value of Proteins of Three BorreliaSpecies (Borrelia burgdorferi Sensu Lato) and Implications for Development and Use of Recombinant Antigens for Serodiagnosis of Lyme Borreliosis in Europe. Clin Diagn Lab Immunol, 1998; 5, 456-62.
35.Magnarelli LA, Ijdo JW, Padula SJ, et al. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J Clin Microbiol, 2000; 38,1735-9.
36.Lefebvre RB, Perng GC, Johnson RC. The 83-kilodalton antigen of Borrelia burgdorferi which stimulates immunoglobulin M (IgM) and IgG responses in infected hosts is expressed by a chromosomal gene. J Clin Microbiol, 1990; 28, 1673-5.
37.Rössler D, Eiffert H, Jauris-Heipke S, et al. Molecular and immunological characterization of the p83/100 protein of various Borrelia burgdorferi sensu lato strains. Med Microbiol Immunol, 1995; 184, 23-32.
38.Rauer S, Kayser M, Neubert U, et al. Establishment of enzyme-linked immunosorbent assay using purified recombinant 83-kilodalton antigen of Borrelia burgdorferi sensu stricto and Borrelia afzelii for serodiagnosis of Lyme disease. J Clin Microbiol, 1995; 33, 2596-600.
39.Luft BJ, Mudri S, Jiang W, et al. The 93-kilodalton protein of Borrelia burgdorferi: an immunodominant protoplasmic cylinder antigen. Infect Immun, 1992; 60, 4309-21.
40.Verma A, Brissette CA, Bowman A, et al. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect Immun, 2009; 77,4940-6.
Biomed Environ Sci, 2016; 29(5): 323-330 10.3967/bes2016.042 ISSN: 0895-3988
Supplementary Materials

Table S1. Oligonucleotides Used of Each Antigen in This Study
*This study was supported by the National Science and Technology Major Project [No. 2013ZX10004-001].
#Correspondence should be addressed to HAO Qin, Professor, Tel: 86-10-58900772, E-mail: haoqin@icdc.cn; WAN Kang Lin, Professor, Tel: 86-10-58900776, E-mail: wankanglin@icdc.cn
Biographical note of the first author LIU Wei, female, born in 1990, master's degree candidate, majoring in serodiagnosis of Lyme borreliosis.
November 5, 2015;
 Biomedical and Environmental Sciences2016年5期
Biomedical and Environmental Sciences2016年5期
- Biomedical and Environmental Sciences的其它文章
- Development of a Novel PmpD-N ELISA for Chlamydia psittaci Infection*
- Viral Etiology Relationship between Human Papillomavirus and Human Breast Cancer and Target of Gene Therapy
- Whole Genome Sequencing and Comparisons of Different Chinese Rabies Virus Lineages Including the First Complete Genome of an Arctic-like Strain in China*
- The Status and Associated Factors of Successful Aging among Older Adults Residing in Longevity Areas in China*
- Cognitive Training in Older Adults with Mild Cognitive Impairment
- Dietary Exposure to Benzyl Butyl Phthalate in China*
