Circulating MicroRNA-21 is Downregulated in Patients with Metabolic Syndrome*
HE Qing Fang, WANG Li Xin, ZHONG Jie Ming, HU Ru Ying, FANG Le, WANG Hao,GONG Wei Wei, ZHANG Jie, PAN Jing, and YU Min
Letter to the Editor
Circulating MicroRNA-21 is Downregulated in Patients with Metabolic Syndrome*
HE Qing Fang, WANG Li Xin, ZHONG Jie Ming, HU Ru Ying, FANG Le, WANG Hao,GONG Wei Wei, ZHANG Jie, PAN Jing, and YU Min#
The microRNA-21 (miR-21) is known to play a major role in cancer progression; however, its function in the cardiovascular system appears to be even more complex and conflicting. To characterize miR-21 expression in the plasma of individuals with or without metabolic syndrome (MetS), 58 MetS cases and 96 non-MetS controls were investigated. Expression levels of miR-21 were significantly decreased in the circulation of MetS subjects (OR=0.52, 95% CI: 0.29-0.92) compared with that of non-MetS subjects. Body mass index (BMI) and the number of MetS components had a negative correlation with the level of miR-21, whereas age was inversely related to the level of miR-21. No significant difference was detected in miR-21 levels between the sexes (P=0.056). MiR-21 might be a negative regulating factor in MetS.
Metabolic syndrome (MetS) is a major public health concern, usually characterized by the clustering of several risk factors, such as central obesity,dyslipidemia, insulin resistance, and hypertension. MicroRNAs (miRNAs) are endogenous, 20-23 nucleotide, small, non-coding RNAs that regulate a variety of target genes involved in cardiovascular physiology and diseases. A large number of stable miRNAs are present in serum and plasma, and dysregulated expression of miRNAs in the serum and plasma has been demonstrated in association with various diseases, including MetS[1-3]. The microRNA-21 (miR-21) gene is located on chromosome 17, in the 10thintron of a protein-encoding gene, TMEM49. MiR-21 has been reported to contribute to the pathogenesis of diseases, potentially by silencing metabolic pathways, targeting antiangiogenic factors,and playing a distinct role in the control of angiogenesis and vascular integrity[4].
Fifty-eight cases who met the criteria of the Chinese Diabetes Society (CDS)[5]and 96 non-MetS controls were recruited into our field investigation. Written informed consent was obtained prior to the investigation. The field investigation included the administration of an epidemiological questionnaire,a physical examination, and laboratory tests. The epidemiological questionnaire obtained data on general demographic characteristics, family history,medical history, smoking and drinking habits, and other lifestyle factors.
Investigators performed physical examinations to obtain the height, weight, waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure (DBP) of participants. The body mass index (BMI) was calculated as follows: BMI = weight (kg)/height2(m2).
A 5 mL fasting venous blood sample was collected from each subject. Plasma was subsequently separated by centrifugation and divided into two parts. One part of plasma was subjected to automated biochemical analysis to measure the levels of total cholesterol (TC),triglycerides (TG), high-density lipoprotein cholesterol (HDLC), and fasting blood glucose (FBG). The other part of plasma was for the miRNA quantification assay. Another 1 mL venous blood sample was collected after a 2 h oral glucose tolerance test (OGTT) to evaluate 2 h postprandial blood glucose (2hPG).
For each sample, total RNA with preserved miRNAs was extracted from 200 µL of plasma using the QIAGEN miRNeasy Serum/Plasma Kit (Qiagen,Dusseldorf, Germany) by a plasma/QIAzol ratio of 1:5. The total RNA concentration was determined using an ultraviolet spectrophotometer and 1% agarose gel electrophoresis. The MiRNeasy Serum/Plasma Spike-In Control (LyophilizedCaenorhabditis elegans miR-39 miRNA mimic) was added to the plasma samples prior to RNA extraction as an external control and miR-16 was used as an internal control for normalization.
Real-time fluorescent quantitative reverse transcription PCR (qRT-PCR) was used to assay the level of microRNAs. The expression of mature miR-16 and miR-21 were detected using the Taqman miRNA qRT-PCR Assay (Applied Biosystems, Carlsbad,CA). RT-PCR was performed in the Applied Biosystems 7500 System in two steps. The RT reaction was performed in a total volume of 15 µL,and the qPCR amplification reaction volume was 20 µL, using the manufacturer's respective standard protocols. Reactions for each RNA sample were performed in duplicate under the same conditions. No-template controls were used throughout the experiment. The data were analyzed using the SDS Relative quantification Software version 2.4 (Applied Biosystems, Inc.). MiR-16 expression value of each sample was used as an internal control for this sample when calculating the relative expression values of the gene of interest, miR-21, and the 2-ΔΔctmethod was used.
All data were analyzed using SPSS 13.0 software. The continuous variables were presented as the means±SD. Relative miRNAs expression levels (non-normal distribution data) were log-transformed into a normal distribution. The univariate logistic regression analysis was performed to assess associations with characteristics between the cases and controls, and the group variable (cases=1,controls=0) was used as the dependent variable (sls=0.1, sle=0.05). Correlations between the expression level of the circulating miR-21 and the individual component of MetS were assessed by Pearson's correlation, and the correlation between the expression level of the circulating miR-21 and the numbers of MetS components was assessed by nonparametric correlations.
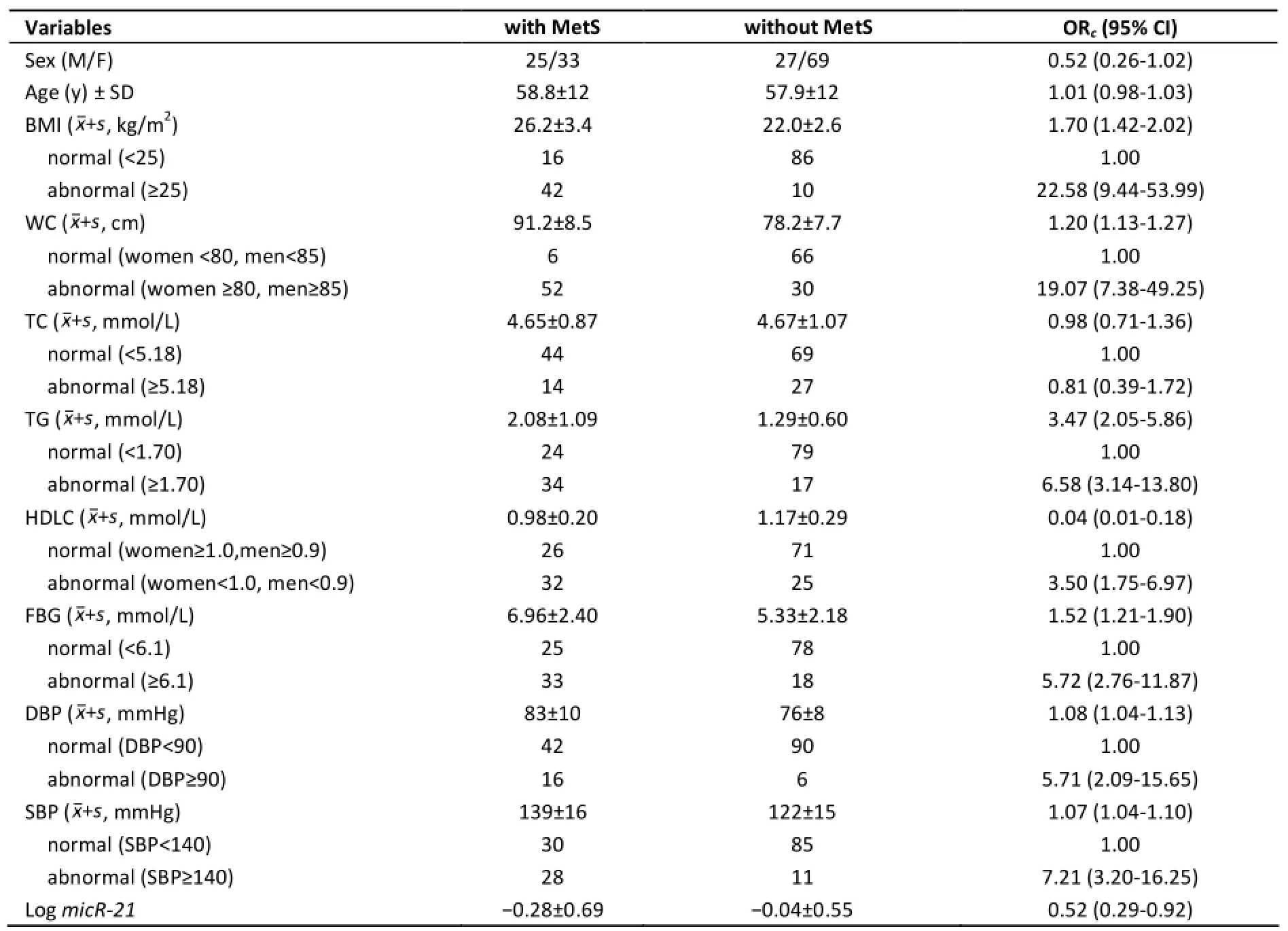
Table 1. Characteristics of Subjects with and without Metabolic Syndrome
The demographic characteristics and the status of MetS in the 154 subjects are shown in Table 1. The distributions of sex, age and TC were of no significant difference between the groups with andwithout MetS. All the MetS components (BMI, TG,HDLC, FBG, SBP, and DBP) together with WC and miR-21 levels showed significant difference between the cases and the controls. MiR-21 levels were significantly elevated in the circulation of the non-MetS subjects (OR=0.52, 95% CI: 0.29-0.92).
Chen[3]also found that miRNAs, including miR-21, could be detected in the serum and plasma of both humans and various animal species, and the expression levels were reproducible and consistent among individuals. However, on the function and mechanism of action of miR-21 in MetS-related diseases, such as obesity, diabetes mellitus, and cardiovascular diseases, controversial reports showed that this miRNA works in complex and unknown ways. The relationship between miR-21 and each individual MetS component in our study was tested (Figure 1). Of the six components (BMI,TG, HDLC, FBG, SBP, DBP) of MetS, BMI was the only factor that exhibited a significant correlation with the level of circulating miR-21 (r=0.258, P=0.001 by Pearson's correlation). The level of circulating miR-21 increased significantly with age (r=0.200,P=0.013 by Pearson's correlation).
Obesity is a prevalent manifestation of the MetS. MiR-21 has been reported to increase adipogenesis by inhibiting the TGF signaling pathway, which is known to inhibit adipogenesis. MiR-21 was also reported to be robustly expressed in human adipose tissue and positively correlated with BMI, but it is still unknown whether miR-21 can induce adipose tissue expansion, or if it increases as a consequence of adipose tissue expansion or the inclusion of additional cell types within the tissue[6]. In disagreement with the above-mentioned reports,our study revealed that BMI was the only factor that exhibited a significant correlation with the level of circulating miR-21 (r=0.258, P=0.001 by Pearson's correlation).
MiR-21 is also found to be downregulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes. It has been proposed that miR-21 could negatively regulate the expression of Fas ligand, MTPN, APAF1, IL12A, IL22, and IL-1B genes[7]. Chen[3]found that diabetic patients had a significantly altered expression profile of serum miRNAs compared with healthy subjects; our study also revealed a negative association between miR-21 and FBG. The reason might be that, in disease states,the miRNAs were released by different organs, but the mechanism is not yet clear.
In the cardiovascular system, miR-21 is reportedto be upregulated under many pathophysiological conditions, contributing to the onset of cardiac fibrosis and dysfunction of circulating angiogenic cells in patients with coronary artery disease[8], and playing a role in the integration of hemodynamics and VEGF signaling during angiogenesis. However,miR-21 was found to inhibit angiogenesis by targeting RhoB expression in endothelial cells. RhoB gene silencing impaired endothelial cell migration and tubulogenesis, thus providing a possible mechanism for miR-21 to inhibit angiogenesis. The results of our study showed no significant association between miR-21 and either DBP or SBP. Taken together, recent reports have demonstrated that the levels of miR-21 were either increased or decreased in affected cardiac tissues and/or plasma;the reason might partly depend on the time point at which the samples were taken, and the regulation of miR-21 expression and function in the cardiovascular system is too complex to explain at this time.
Consistent with our study, an investigation by Sun et al.[9]into miR-21 expression in non-alcoholic fatty liver disease (NAFLD), both in vivo and in vitro,also revealed that the serum levels of miR-21 were lower in patients with NAFLD than in healthy controls. Furthermore, results from in vitro experiments revealed that miR-21 decreased the levels of TG and TC in PA/OA-treated HepG2 cells. This suggested that miR-21 regulated TG and TC metabolism in NAFLD, and that this effect was achieved by the inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) expression. The expression of miR-21 was also found to be decreased in the livers of mice fed a high-fat diet and in Hepa 1-6 cells treated with stearic acid (SA).
Though miR-21 has been shown to be downregulated in MetS, our results should be interpreted with caution. First, the case-control study design has a limited ability to investigate the causal roles of miR-21 in the developments of MetS. Second, the small sample size of this study might limit its statistical power. Third, the BMI cutoff and the time-, cell-, and disease-specific differences should be taken into account in the future development of miR-21-based approaches in MetS. Fourth, miR-21 targets different genes in different cell types. Fifth, although CDS criteria might be more suitable for the Chinese population, its BMI cut-off is lower than World Health Organization (WHO)'s. These discrepancies between studies reflect the challenges inherent in studies on the role of miR-21 in MetS; thus, further studies would be necessary to establish the precise role of miR-21 in MetS.
Circulating microRNAs are promising biomarkers for noninvasive testing and dynamic monitoring in patients with chronic diseases. The use of qRT-PCR based on Taqman is the most frequent approach for estimating circulating miRNAs in the present. Normalization is critically important to correct inter-sample variation due to differences in reaction efficiencies or sample preparation. However, no standard endogenous control for normalizing the quantification of circulating microRNAs exists,making the results incomparable. Currently,synthetic miRNA molecules, used as spike-in controls,are added to a sample before RNA extraction, and RNA molecules U6, RNU6B, 18S and miR-16 are used as internal controls to normalize target miRNA expression data. In this study, we have adopted miR-16 as an internal control from literature, as it is expressed at high levels in plasma and serum that are relatively invariant across a large number of samples[10].
#Correspondence should be addressed to YU Min,Chief Physician, Master, Tel: 86-571-87115005, E-mail:myu@cdc.zj.cn
Biographical note of the first author: HE Qing Fang,female, born in 1972, master of science in agriculture,chief technician, focus on molecular epidemiology of chronic disease's study.
Accepted: May 1, 2016
REFERENCES
1. Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res,2012; 93, 583-93
2. Karolina DS, Tavintharan S, Armugam A, et al. Circulating miRNA Profiles in Patients with Metabolic Syndrome. J Clin Endocrinol Metab, 2012; 97, E2271-6.
3. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a noval class of biomarker for diagnosis of cancer and other diseases. Cell Res, 2008; 18, 997-1006
4. Bátkai S, Thum T. MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep, 2012; 14, 79-87.
5. Chinese Medical Association Diabetes Society Metabolic Syndrome group. The suggestion of the diagnostic criteria of metabolic syndrome by Chinese Medical Association Diabetes Society. Chinese Journal of Diabetes Mellitus, 2004; 12, 156-61. (In Chinese)
6. Keller P, Gburcik V, Petrovic N, et al. Gene-chip studies of adipogenesis-regulated microRNAs in mouse primary adipocytes and human obesity. BMC Endocrine Disorders,2011; 11, 7.
7. Salas-Pérez F, Codner E, Valencia E, et al. MicroRNAs miR-21a and miR-93 are down regulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes. Immunobiology, 2013; 218, 733-7
8. Fleissner F, Jazbutyte V, Fiedler J, et al. Asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery through a microRNA-21 dependent mechanism. Circ Res, 2010; 107, 138-43.
9. Sun C, Huang F, Liu X, et al. MiR-21 regulates triglyceride and cholesterol metabolism innon-alcoholic fatty liver disease by targeting HMGCR. Int J Mol Med, 2015; 35, 847-53.
10.Hu J, Wang Z, Liao BY, et al. Human miR-1228 as a stable endogenous control for the quantification of circulating microRNAs in cancer patients. Int J Cancer, 2014; 135, 1187-94.
10.3967/bes2016.050
*This paper was supported by Qianjiang Talents Project of Science Technology Department of Zhejiang Province (project number 2013R10078) (http://www.zjkjt.gov.cn/), and by Medical and Health Science Research Fund of Zhejiang Province (project number 2013KYB053, 2008A034, 2007A035, 2006A019)(http://www.zjwst.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Zhejiang Provincial Center for Disease Prevention and Control, Hangzhou 310051, Zhejiang, China
February 24, 2016;
 Biomedical and Environmental Sciences2016年5期
Biomedical and Environmental Sciences2016年5期
- Biomedical and Environmental Sciences的其它文章
- Development of a Novel PmpD-N ELISA for Chlamydia psittaci Infection*
- Evaluation of Six Recombinant Proteins for Serological Diagnosis of Lyme Borreliosis in China*
- Viral Etiology Relationship between Human Papillomavirus and Human Breast Cancer and Target of Gene Therapy
- Whole Genome Sequencing and Comparisons of Different Chinese Rabies Virus Lineages Including the First Complete Genome of an Arctic-like Strain in China*
- The Status and Associated Factors of Successful Aging among Older Adults Residing in Longevity Areas in China*
- Cognitive Training in Older Adults with Mild Cognitive Impairment
