AMPK调控Ca2+内流对高糖诱导内皮细胞凋亡的作用及其机制研究
卢婷,郜攀,司良毅,赵坤
·基础研究·
AMPK调控Ca2+内流对高糖诱导内皮细胞凋亡的作用及其机制研究
卢婷,郜攀,司良毅,赵坤
目的观察AMP依赖的蛋白激酶(AMPK)对高糖刺激内皮细胞凋亡的抑制作用,并初步探讨其机制。方法体外培养MS-1内皮细胞株,分别用AMPK激动剂、AMPK抑制剂、钙库依赖性钙离子通道(SOCC)抑制剂2-APB 和(或)高糖处理,另设对照组(未经任何方式干预)。采用TUNEL法检测细胞凋亡情况,激光共聚焦显微镜检测细胞内钙离子(Ca2+)内流,Western blotting检测SOCC蛋白Stim1和Orai1的表达。结果与对照组比较,高糖能够明显诱导内皮细胞凋亡,增加Stim1和Orai1蛋白表达(P<0.05)。与高糖组比较,AMPK抑制剂+高糖能够明显增强高糖诱导的内皮细胞的凋亡(P<0.05),而AMPK激动剂+高糖能够明显抑制高糖诱导的内皮细胞凋亡,并降低Stim1和Orai1蛋白表达(P<0.05)。与对照组比较,高糖能够明显诱导内皮细胞Ca2+内流;与高糖组比较,2-APB+高糖能够明显抑制高糖诱导的内皮细胞Ca2+内流,并阻断高糖对内皮细胞凋亡的诱导作用,而AMPK激动剂能够明显抑制高糖诱导的内皮细胞Ca2+内流。结论AMPK能够通过降低Stim1和Orai1蛋白的表达,抑制SOCC介导的Ca2+内流,进而阻断高糖刺激的内皮细胞凋亡,对内皮细胞功能起重要的保护作用。
AMP活化蛋白激酶类;钙离子载体;高血糖症;钙库依赖性钙离子通道;内皮细胞
随着中国步入老年化社会,冠心病(CHD)的发病率和致死率逐年增高[1]。动脉粥样硬化(atherosclerosis,AS)是CHD的病理学基础[2],其中血管壁的内皮细胞(endothelial cells,ECs)在血管稳态平衡中具有重要作用,研究证实ECs受损和动脉粥样硬化的多种传统危险因素均可诱导ECs凋亡,而ECs凋亡可能是动脉粥样硬化病理发生的早期事件,并可促进粥样硬化病变形成、斑块侵蚀和急性冠脉综合征形成[3]。
AMP依赖的蛋白激酶(adenosine 5'-monophosphateactivated protein kinase,AMPK)是生物能量代谢调节的关键分子[4],本课题组在先前的实验中发现抑制AMPK的活性能够诱导高糖对内皮细胞的损伤[5]。近年研究发现,一种新型的钙离子通道——钙库依赖性钙离子通道(store operated Ca2+channel,SOCC)在调控细胞内钙离子内流中发挥重要作用[6],尤其是SOCC调节的钙离子内流在调控细胞周期、分化、增殖和凋亡中具有非常重要的作用。SOCC的主要组成成分是两个相互作用的分子Orai1和Stim1,研究发现抑制SOCC蛋白表达可以抑制神经细胞凋亡[7]。本实验拟观察在高糖刺激下,AMPK对内皮细胞凋亡的保护作用并初步探讨SOCC通道在此过程中的作用。
1 材料与方法
1.1 主要材料及试剂 内皮细胞采用ATCC细胞库的小鼠胰岛微血管内皮细胞株MS-1(ATCC® CRL-2460TM,美国)。DMEM培养基、胎牛血清(FBS)购自美国Gibco公司,HBSS缓冲液和PBS缓冲液购自北京鼎国生物试剂公司,TUNEL凋亡检测试剂盒购自瑞士Roche公司,Stim1、Orai1一抗抗体购自英国Abcam公司,内参GAPDH一抗、辣根过氧化物酶标记的兔抗山羊IgG购自美国Santa Cruz公司,RIPA蛋白裂解液购自北京鼎国生物公司,其余试剂包括AMPK抑制剂和激动剂等均购自日本Sigma公司。
1.2 细胞培养 将购买的MS-1内皮细胞迅速用20% FBS溶解,1000r/min离心后得到的沉淀再次用含有5% FBS的DMEM培养基溶解,分装到培养瓶中,在37℃、5%CO2培养孵育箱中进行培养。以复苏传代后3~5代MS-1细胞株进行实验,0.25%胰蛋白酶消化制备细胞悬液,调整细胞密度为5×107/L,接种于培养板备用。经37℃、5%CO2、5%FBS预培养24h和0.5%胎牛血清培养基预培养处理12h后进行分组。
1.3 细胞分组及凋亡检测 为明确AMPK对高糖诱导内皮细胞凋亡的作用,将细胞分为6组:对照组(未经任何方式干预)、高糖组(30mmol/L葡萄糖处理48h)、高糖+AMPK抑制剂组(30mmol/L葡萄糖处理48h+20μmol/L复合物C预处理1h)、高糖+AMPK激动剂组(30mmol/L葡萄糖处理48h+1mmol/L AICAR预处理1h)、复合物C组(20μmol/L复合物C预处理1h)和AICAR组(1mmol/L AICAR预处理1h)。为进一步明确高糖诱导的钙内流对内皮细胞凋亡的影响,将细胞分为4组:对照组、高糖组、高糖+SOCC抑制剂(2-APB)组(30mmol/L葡萄糖处理48h+75μmol/L 2-APB预处理1h)、2-APB组(75μmol/ L 2-APB预处理1h)。细胞经胰酶消化后,制成细胞爬片,按照Roche凋亡试剂盒说明书方法进行,将载玻片取出置于水平面,用滤纸小心吸去多余液体,将细胞用4%多聚甲醛(溶于pH7.4的PBS中)在室温下固定30min,PBS漂洗5次;浸入封闭液(3%H2O2溶于甲醇)中,室温封闭10min后再用PBS漂洗5min×3次;浸入细胞膜通透液中,室温下反应30s~2min。采用TUNEL凋亡试剂盒中的标记物标记凋亡的细胞核,以每100个细胞中的TUNEL凋亡细胞数表示结果。
1.4 细胞分组及钙离子内流检测 为明确高糖诱导的钙内流与SOCC通道的关系,将细胞分为4组:对照组、高糖组、高糖+2-APB组和2-APB组。为进一步明确AMPK对高糖诱导的钙内流的影响,将细胞分为4组:对照组、高糖组、高糖+AICAR组和AICAR组。将内皮细胞培育在HEPES缓冲液中,采用激光共聚焦体系检测胞质内Ca2+探针Fura-2/AM荧光强度。细胞在6孔板中以1% FBS孵育2h,用HBSS缓冲液洗1次,加入3μmol/L的Fura-2/AM荧光探针孵育30min,用HBSS缓冲液清洗1次。采用卡尔蔡司激光共聚焦显微镜(Carl Zeiss GmbH,德国)采集荧光探针信号,双激发波长为340、380nm,发射波长为520nm。其中毒胡萝卜素(thapsigargin,TG,实验浓度1μmol/L)为诱导内质网钙库释放进而激活SOCC钙离子通道的SOCC激动剂,于实验第2分钟时加入。最初3min内,培养基中无Ca2+([Ca2+]0),此后于第3分钟后开始加入1.5mmol/ L CaCl2([Ca2+]1.5)至反应液中,每隔10s采集一次荧光信号,采用Zen2012软件系统检测各个时间点的荧光强度并绘制曲线。
1.5 Western blotting检测细胞内SOCC蛋白表达细胞分4组:对照组、高糖组、高糖+AICAR组和AICAR组。收集各组细胞后,以RIPA法裂解细胞,采用考马斯亮蓝法进行蛋白定量。取约40μg总蛋白,置于95℃沸水中变性5min,采用5%浓缩胶和10%分离胶行SDS聚丙烯酰胺凝胶电泳,电泳条件恒压80mV,时间为3h。然后采用三明治法将蛋白转移至PVD膜,转膜采用Bio-Rad电转仪,转膜条件为恒流15mA,时间为90min。之后将PVDF膜用5%脱脂奶粉封闭1h,加入封闭液稀释的一抗,山羊抗小鼠Stim1多克隆抗体(1:200稀释)和山羊抗小鼠Orai1多克隆抗体(1:200稀释),封闭过夜,加辣根过氧化物酶标记的兔抗山羊IgG(稀释浓度1:500)室温反应1h后染色。用相同方法检测内参GAPDH蛋白表达(稀释浓度1:1000)。用FR-200凝胶成像分析系统进行定量分析。
1.6 统计学处理 采用SPSS 19.0软件进行分析。计量资料以表示,比较其方差齐性,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。P<0.05为差异有统计学意义。
2 结 果
2.1 AMPK对高糖诱导内皮细胞凋亡的抑制作用体外培养MS-1内皮细胞株,预先用复合物C或AICAR预处理,再加入高糖刺激内皮细胞,TUNEL法检测细胞的凋亡情况。结果显示,与对照组比较,高糖刺激后内皮细胞凋亡显著增加(P<0.05);与高糖刺激组比较,AICAR能显著抑制高糖刺激的内皮细胞凋亡(P<0.05),复合物C则进一步诱导内皮细胞凋亡(P<0.05,图1)。
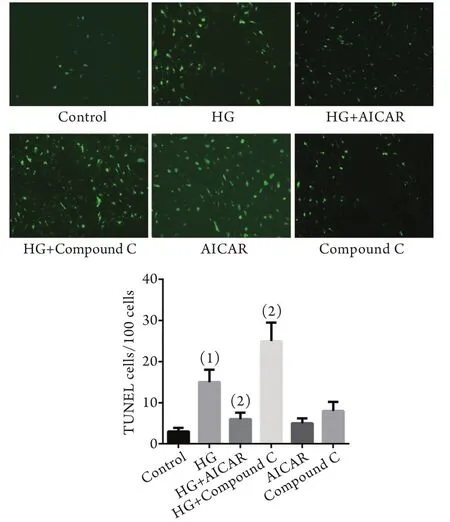
图1 AMPK对高糖诱导内皮细胞凋亡的影响(TUNEL ×10)Fig.1 Effects of AMPK on high glucose-stimulated endothelial cell apoptosis (TUNEL ×10)HG. High glucose; AICAR. 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (an AMPK agonist). (1)P<0.05 compared with control group; (2)P<0.05 compared with HG group
2.2 高糖诱导细胞钙离子内流([Ca2+]i)对内皮细胞凋亡的影响 将内皮细胞予以2-APB预处理后,再予以高糖刺激。结果显示,与对照组相比,高糖刺激可明显诱导细胞内SOCC介导的Ca2+内流,而与高糖组相比,2-APB则能够明显抑制高糖诱导的Ca2+内流(P<0.05)。另一方面,高糖诱导的内皮细胞凋亡也可被2-APB明显抑制(P<0.05,图2)。

图2 内皮细胞[Ca2+]i对内皮细胞凋亡的影响Fig.2 Effects of calcium influx on the apoptosis of endothelial cellsHG. High glucose; 2-APB. 2-aminoethyl diphenylborinate (a blocker of store-operated Ca2+channels). A. Effects of SOCC blocker (2-APB) on high glucose-induced calcium influx; B. Effects of SOCC blocker (2-APB) on high glucose-induced endothelial cell apoptosis; C. Column chart of endothelial cell apoptosis (TUNEL). (1)P<0.05 compared with control group; (2)P<0.05 compared with HG group
2.3 AMPK对高糖诱导的[Ca2+]i的影响 细胞予AMPK激动剂AICAR(1mmol/L)预处理1h,再加入30mmol/L高糖刺激4h,细胞分成4组:对照组、高糖组、高糖+AICAR组、AICAR组。结果显示,高糖诱导的Ca2+内流被AMPK激动剂AICAR显著抑制(P<0.05,图3)。
2.4 AMPK对高糖诱导的内皮细胞SOCC通道蛋白表达的抑制作用 细胞予AICAR预处理后,再予高糖处理,检测各组SOCC通道蛋白分子Stim1和Orai1蛋白表达情况。与对照组比较,高糖刺激可明显增加Stim1和Orai1蛋白的表达,而AICAR则能够明显抑制Stim1、Orai1蛋白的表达(P<0.05,图4)。
3 讨 论
CHD是老年人的常见病和多发病,也是老年患者临床死亡的重要原因,其病理基础为冠状动脉粥样硬化。研究发现,内皮细胞能够释放大量活性因子,如一氧化氮、血管紧张素和内皮素等,可调节冠脉事件中的血栓形成、炎症和免疫反应[8-10]。血管内皮在外界因素作用下出现损伤、凋亡,可使血管平滑肌直接暴露于血液中的脂蛋白、活性氧等刺激因素,推动血管内皮细胞的异常修复[11-12],最终导致动脉粥样硬化的发生[13]。
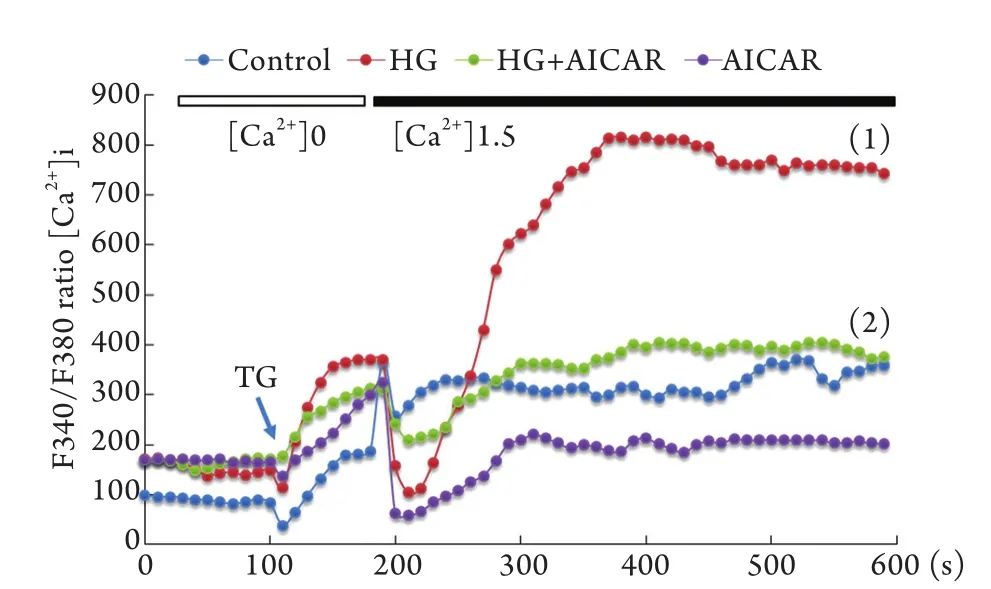
图3 AMPK对高糖诱导的[Ca2+]i的抑制作用Fig.3 Inhibitive effect of AMPK on high glucose-induced Ca2+influx to endothelial cellsHG. High glucose; AICAR. 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (an AMPK agonist); TG. Thapsigargin; (1)P<0.05 compared with control group; (2)P<0.05 compared with HG group
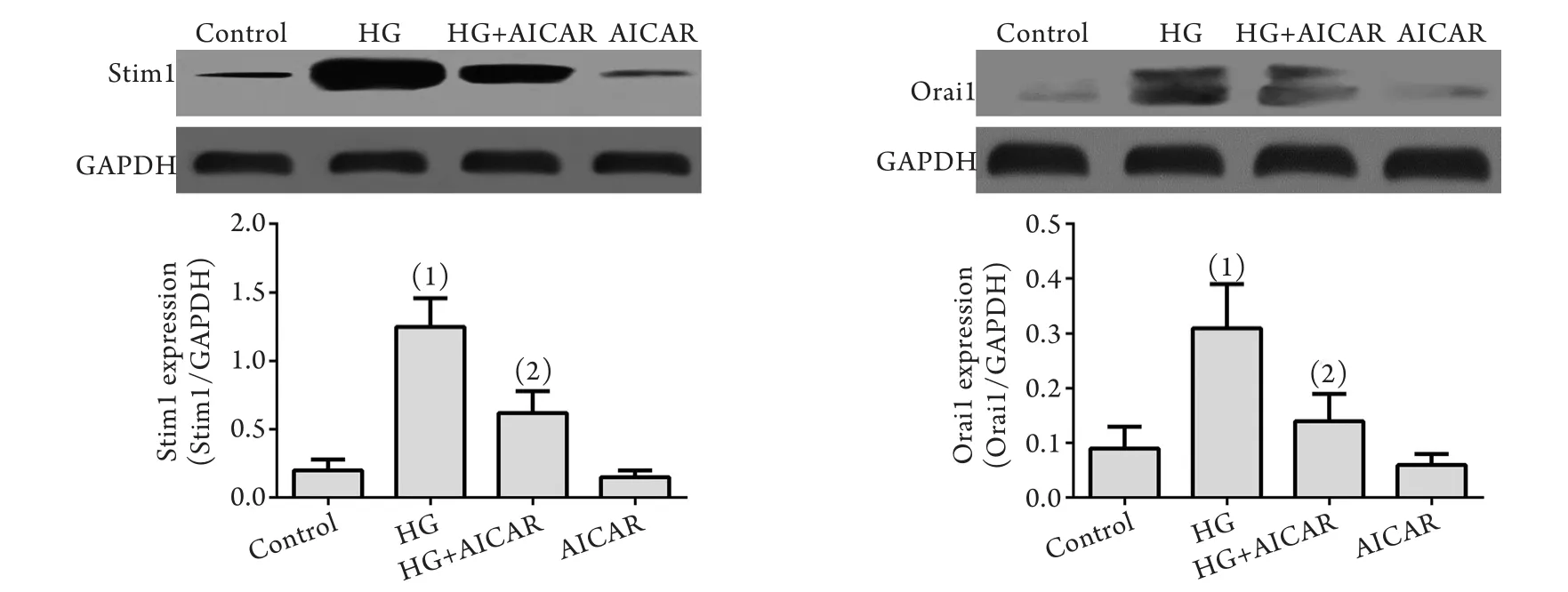
图4 AMPK对高糖诱导的内皮细胞SOCC通道Stim1、Orai1蛋白表达的影响(Western blotting)Fig.4 Effects of AMPK on the expressions of high glucose-stimulated expression of SOCC proteins (Stim1 and Orai1) in endothelial cells (Western blotting)HG. High glucose; AICAR. 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (an AMPK agonist). (1)P<0.05 compared with control group; (2)P<0.05 compared with HG group
Ca2+对维持细胞正常生理功能具有重要作用,一方面可作为第二信使介导细胞内多种生物学反应,另一方面Ca2+稳态在维持细胞的增殖、分化和凋亡中发挥枢纽作用[14-15]。研究表明,血管内皮细胞外的Ca2+经细胞膜上的钙通道流入细胞内,导致细胞内Ca2+超载,造成血管内皮细胞损伤;毒胡萝卜素通过抑制内质网钙-ATP酶、排空内质网Ca2+诱导细胞严重的内质网应激反应,导致内皮细胞凋亡[16]。本研究中,高糖刺激内皮细胞后Ca2+内流明显增加,而2-APB作为SOCC通道抑制剂在抑制高糖诱导内皮细胞Ca2+内流的同时,还可抑制高糖诱导的内皮细胞凋亡,提示内皮细胞Ca2+内流能够明显诱导内皮细胞凋亡的发生。
AMPK是一个含有丝/苏氨酸蛋白激酶活性的异源三聚体,由催化亚基α、调节亚基β和γ通过不同的排列和空间构象形成。既往本课题组在观察白藜芦醇(一种具有抗氧化、抗炎、抗凋亡等作用的多酚类化合物)拮抗高糖致内皮细胞损害的研究中发现,抑制AMPK活性后,白藜芦醇对内皮细胞功能的保护作用显著降低,说明AMPK在内皮细胞功能的维持中发挥重要作用[5,17]。SOCC是一种可持续诱导Ca2+内流的钙通道,可调节细胞的基因转录、生长和分化等。SOCC的作用机制为:当外界或内在刺激使胞内钙库中Ca2+释放,使钙库中Ca2+浓度下降到一定程度时,细胞膜上SOCC开放产生Ca2+内流,引起细胞内Ca2+浓度持续缓慢上升,从而补充胞质和钙库中的Ca2+[18]。目前研究发现,Stim1和Orai是内皮细胞最主要的SOCC蛋白成分[19-20]。本实验首先观察了AMPK激动剂AICAR和高糖刺激对钙通道蛋白表达的影响,结果显示高糖能够促进Stim1和Orai1蛋白的表达,而AICAR则能够显著抑制高糖诱导的Stim1和Orai1蛋白表达,进一步研究发现AICAR可显著抑制高糖诱导的Ca2+内流,结果提示激动AMPK后能够显著抑制高糖诱导的内皮细胞凋亡,其机制可能与抑制SOCC介导的Ca2+内流密切相关。
综上所述,内皮细胞凋亡在动脉粥样硬化发病中具有重要作用,而AMPK能够通过抑制SOCC介导的Ca2+内流而抑制内皮细胞凋亡,对内皮细胞功能起到重要的保护作用。后续研究应观察在动物模型中AMPK对内皮细胞凋亡的影响,并进一步明确AMPK在抑制内皮细胞凋亡时发挥作用的具体亚单位。
[1]He LY, Zhao JF, Han JL,et al. Correlation between serum free fatty acids levels and Gensini score in elderly patients with coronary heart disease[J]. J Geriatr Cardiol, 2014, 11(1): 57-62.
[2]Liang B, Qi Z, Ding HY,et al. Correlation study of B type natriuretic peptide and severity of coronary artery lesions and cardiac function[J]. Chin J Pract Intern Med, 2015, 35(3): 257-258.[梁滨, 戚真, 丁怀玉, 等. B型利钠肽与冠状动脉病变程度及心功能相关性研究[J]. 中国实用内科杂志, 2015, 35(3): 257-258.]
[3]Riwanto M, Rohrer L, Roschitzki B,et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling[J]. Circulation, 2013, 127(8): 891-904.
[4]Song SZ, Zhang DM, Fu YQ,et al. Expression of adiponectin and AMPK mRNA in pregnant rats with high sucrose and fat diet[J]. J Zhengzhou Univ (Med Sci), 2012, 47(3): 309-312.[宋帅召,张东铭, 付艳芹, 等. 高糖高脂饮食妊娠大鼠脂联素和AMPK mRNA的表达[J]. 郑州大学学报(医学版), 2012, 47(3): 309-312.]
[5]Xu Q, Si LY. Protective effects of AMP-activated protein kinase in the cardiovascular system[J]. J Cell Mol Med, 2010, 14(11): 2604-2613.
[6]Elvers M, Herrmann A, Seizer P,et al. Intracellular cyclophilin A is an important Ca(2+) regulator in platelets and critically involved in arterial thrombus formation[J]. Blood, 2012, 120(6): 1317-1326.
[7]Zhang M, Song JN, Wu Y,et al. Suppression of STIM1 in the early stage after global ischemia attenuates the injury of delayed neuronal death by inhibiting store-operated calcium entryinduced apoptosis in rats[J]. Neuroreport, 2014, 25(7): 507-513.
[8]Kurokawa H, Sugiyama S, Nozaki T,et al. Telmisartan enhances mitochondrial activity and alters cellular functions in human coronary artery endothelial cellsviaAMP-activated protein kinase pathway[J]. Atherosclerosis, 2015, 239(2): 375-385.
[9]Qin Q, Chen M, Yi B,et al. Orphan nuclear receptor Nur77 is a novel negative regulator of endothelin-1 expression in vascular endothelial cells[J]. J Mol Cell Cardiol, 2014, 77: 20-28.
[10] Yang GJ. An analysis of benefits of aspirin therapy in cardiovascular primary prevention[J]. Chin J Pract Intern Med, 2012, 32(4): 274-276.[杨国君. 阿司匹林一级预防获益机制新解[J]. 中国实用内科杂志, 2012, 32(4): 274-276.]
[11] Ma R, Ren J, Men JL. Study on coagulation function in patients with chronic heart failure[J]. Tianjin Med J, 2013, 41(1): 65-66.[马睿, 任静, 门剑龙. 慢性心力衰竭患者凝血功能的研究[J]. 天津医药, 2013, 41(1): 65-66.]
[12] Fan QB, Jian LG. Effects of different doses of atorvastatin on patients with acute myocardial infarction treated with PCI serum osteopontin[J]. J Zhengzhou Univ (Med Sci), 2012, 47(6): 869-870.[樊清波, 简立国. 不同剂量阿托伐他汀对急性心肌梗死急诊行PCI患者血清骨桥蛋白的影响[J]. 郑州大学学报(医学版), 2012, 47(6): 869-870.]
[13] Stamatelopoulos K, Georgiou S, Kanakakis I,et al. Circulating levels of TNF-like cytokine 1A correlate with reflected waves and atherosclerosis extent and may predict cardiac death in patients with stable coronary artery disease[J]. Cytokine, 2015, 72(1): 102-104.
[14] Uhlig S, Yang Y, Waade J,et al. Differential regulation of lung endothelial permeabilityin vitroandin situ[J]. Cell Physiol Biochem, 2014, 34(1): 1-19.
[15] Moccia F, Lodola F, Dragoni S,et al. Ca2+signalling in endothelial progenitor cells: a novel means to improve cellbased therapy and impair tumour vascularization[J]. Curr Vasc Pharmacol, 2014, 12(1): 87-105.
[16] Tomas-Martin P, Lopez-Guerrero AM, Casas-Rua V,et al. Phospho-STIM1 is a downstream effector that mediates the signaling triggered by IGF-1 in HEK293 cells[J]. Cell Signal, 2015, 27(3): 545-554.
[17] Xu Q, Si LY. Resveratrol role in cardiovascular and metabolic health and potential mechanisms of action[J]. Nutr Res, 2012, 32(9): 648-658.
[18] Shim AH, Tirado-Lee L, Prakriya M. Structural and functional mechanisms of CRAC channel regulation[J]. J Mol Biol, 2015, 427(1): 77-93.
[19] Moccia F, Dragoni S, Lodola F,et al. Store-dependent Ca(2+) entry in endothelial progenitor cells as a perspective tool to enhance cell-based therapy and adverse tumour vascularization[J]. Curr Med Chem, 2012, 19(34): 5802-5818.
[20] Zhou MH, Zheng H, Si H,et al. Stromal interaction molecule 1 (STIM1) and Orai1 mediate histamine-evoked calcium entry and nuclear factor of activated T-cells (NFAT) signaling in human umbilical vein endothelial cells[J]. J Biol Chem, 2014, 289(42): 29446-29456.
Effects of AMPK on high glucose stimulated apoptosis of endothelial cellsviaregulation of calcium influx
LU Ting, GAO Pan, SI Liang-yi*, ZHAO Kun
Department of Geriatrics, Xinan Hospital, Third Military Medical University, Chongqing 400038, China
*< class="emphasis_italic">Corresponding author, E-mail: doctorsly@126.com
, E-mail: doctorsly@126.com
This work was supported by the National Natural Science Foundation of China (81370446, 81370007)
ObjectiveTo investigate the inhibitory effect of adenosine monophosphate (AMP)-dependent protein kinase (AMPK) on high glucose-stimulated endothelial cell apoptosis and its mechanism.MethodsMS-1 endothelial cells were culturedin vitro, and they were treated with AMPK agonist, AMPK inhibitor, 2-APB (a blocker of store operated Ca2+channel (SOCC)) and (or) high glucose, and a control group without any intervention were set up. TUNEL assay was performed to determine apoptotic cells. Laser scanning confocal microscopy was used to assess the Ca2+influx into cells, and Western-blotting was performed to determine the expressions of Stim1 and Orai1 of the store operated Ca2+channel (SOCC) proteins.ResultsApoptosis of endothelial cells was induced significantly, and the expressions of Stim1 and Orai1 were upregulated in high glucose group compared with that in control group (P<0.05). The rate of apoptosis of high glucose-induced endothelial cell was found to be increased in AMPK inhibitor group and decreased in AMPK agonist group, and the expressions of Stim1 and Orai1 were found to be downregulated in AMPK agonist group as compared with that in high glucose group (P<0.05). Compared with the control group, high glucose stimulation significantly induced the Ca2+influx to endothelial cells; compared with high glucose group, 2-APB significantly inhibited high glucose-induced Ca2+influx to endothelial cells, and blocked the inducing effect of high-glucose on endothelial cell apoptosis. Compared with high glucose group, AMPK agonist significantly inhibited high glucose-induced cell Ca2+influx.ConclusionBy reducing the expressions of Stim1 and Orai1, AMPK may inhibit SOCC-mediated Ca2+influx, and block the high glucose-stimulated endothelial cell apoptosis, thus play an important protective role in sustaining endothelial cell function.
AMP-activated protein kinases; calcium ionophores; hyperglycemia; store operated Ca2+channel; endothelial cells
R345
A
0577-7402(2015)10-0773-05
10.11855/j.issn.0577-7402.2015.10.01
2015-03-23;
2015-07-12)
(责任编辑:张小利)
国家自然科学基金(81370446、81370007)
卢婷,硕士研究生。主要从事老年心血管病方面的研究
400038 重庆 第三军医大学西南医院老年病科(卢婷、郜攀、司良毅、赵坤)
司良毅,E-mail:doctorsly@126.com

